Functional holey graphene oxide: a new electrochemically transformed substrate material for dopamine sensing†
A. B. M. Zakariaa,
Erick S. Vasquezb,
Keisha B. Waltersc and
Danuta Leszczynska*d
aDepartment of Chemistry and Biochemistry, Jackson State University, Jackson, MS 39217, USA
bDepartment of Chemical and Materials Engineering, University of Dayton, Dayton, OH 45469, USA. E-mail: evasquez1@udayton.edu
cDave C. Swalm School of Chemical Engineering, Mississippi State University, Mississippi State, MS 39762, USA. E-mail: kwalters@che.msstate.edu
dDepartment of Civil and Environmental Engineering, Jackson State University, Jackson, MS 39217, USA. E-mail: danuta.leszczynska@jsums.edu; Fax: +1-601-979-3238; Tel: +1-601-979-1091
First published on 7th December 2015
Abstract
Increasing active sites through generating holes within the basal plane of graphene sheets is an effective strategy to enhance catalytic performance in various applications such as sensors, electrocatalysis, and electronics. In this study, we report a simple two-step electrochemical approach to convert graphene oxide (GO) into holey graphene oxide (HGO)—graphene sheets with holes ranging from several to tens of nanometers in diameter. The resultant HGO graphene has an order of magnitude more effective surface area than GO, and behaves almost as a reversible electrode system in terms of peak-to-peak seperation value (ΔE) and heterogeneous electron transfer rate constant (k0) towards the Fe(CN)63−/4− redox probe. Characterization of the HGO surface using atomic force microscopy (AFM), transmission electron microscopy (TEM), scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, and cyclic voltammetry confirmed generation of holes on the graphene sheets. β-Cyclodextrin (β-CD) was immobilized on ‘as prepared’ HGO demonstrating an additional advantage from the presence of oxygen-containing functional groups on the resultant HGO surface. The β-CD-HGO nanocomposite was investigated as a potential dopamine (DA) sensor material using amperometric techniques. The linear range for DA detection was 0.1–800 μM (N = 3), sensitivity was 4.4 nA μM−1 cm−2, and the detection limit was 7.6 nM (S/N = 3). In addition to enhanced catalytic performance, HGO can be easily modified with materials such as β-cyclodextrin, as well as nanoparticles, bioactive molecules, and stimuli responsive polymers, providing a promising sensor platform.
Introduction
In recent years, graphene has been a material widely investigated across diverse scientific disciplines, both in academic research and technological applications, due to its unique electronic, mechanical and optical properties.1–3 As a two-dimensional single layer of carbon atoms, the surfaces play a major role in determining its fundamental properties.4 The most common graphene surfaces are comprised of graphene oxide (GO), a nonconductive hydrophilic carbon material produced from exfoliation of graphite oxide.5 Hummer's method is widely utilized for large-scale syntheses of graphene oxide,6 but this method introduces oxygen-containing functional groups—such as epoxy, carboxyl, carbonyl, hydroxyl and ether groups—by disruption of the aromatic lattice in GO in uncontrolled manner.7 This inevitable introduction of functional groups through over-oxidation creates defect sites on sp2 carbon plane of graphene surface and makes GO to be less conductive. To overcome this limitation, researchers have attempted removal of oxygen functional groups from the GO surface and also synthesis of reduced graphene oxide (RGO), a hydrophobic material with large surface area and high conductivity.8 However, RGO suffers from severe drawbacks limiting its application including the inability to be dispersed in water resulting from the loss of the polar, oxygen-containing functional from the surface.Recently, considerable focus has been placed on developing unique morphologies of GO by creating oxidative pores within the basal plane of graphene sheets, with the resultant material known as holey graphene oxide (HGO). A unique advantage of HGO is that it has significantly higher catalytic centers due to the increase in edges associated with holes, as compared to GO and RGO. James et al. reported a promising strategy where gold nanoparticles (AuNPs) were employed to catalyze the photolytic decomposition of H2O2, thus providing a hydroxyl radical to oxidize RGO, and finally resulting in the production of HGO.9 Gregg et al. demonstrated the enzymatic oxidation of GO and RGO to produce holes on the basal plane of graphitic materials.10 Recently, Yi et al. reported bulk preparation of “holey graphenes” using the deposition of Ag nanoparticles in graphene sheets followed by thermal treatment in air.11 However, all of these reported methods are complex, requiring a relatively lengthy synthesis timeframes and precise control over reaction and processing parameters. Hence, there is still a need accurate control over hole formation in the graphene plane using simple, green, and low-cost approaches.
Electrochemical methods are effective ways to modify electronic states by adjusting the electric field to change the Fermi energy level of electrode materials.12 The inexpensive, time-saving, and environmental friendly characteristics of electrochemical approaches has triggered significant interest in their use to tune the properties of carbon nanomaterials (e.g., carbon nanotubes, graphene) to enhance performance in various applications.13 For instance, Dhanraj et al. reported a remarkable transformation of carbon nanotubes (CNTs) to nanoribbons by a two-step electrochemical approach.14 Similarly, Zhou et al. reported that electrochemically prepared reduced graphene oxide (ERGO) sheets displayed a more conducting nature than those prepared by chemical reduction of graphene sheets.15 Electrochemical methods provide an effective means for converting GO to a suitable electrode material with large surface area and high conductivity. To gain full advantage of this method depends on the fabrication process for producing thin films of graphene on conducting substrates. For example, self-assembly,16 spray-coating,17 spin-coating,18 direct drop casting of graphene oxide,19 drop-casting of GO followed by electrochemical reduction,20 and horizontal-dip coating of GO on indium-tin-oxide21 are commonly used fabrication methods. Among these methods, self-assembled monolayers (SAMs) are particularly to control the thickness and uniformity of the graphene films.
β-Cyclodextrin (β-CD) is a cyclic oligosaccharide consisting of seven glucose units, that is toroidal in shape with a hydrophobic inner cavity and a hydrophilic exterior.22 β-CD is a well known biocompatible supramolecule that binds selectively various organic, inorganic, and biological guest molecules in the inner cavity. Moreover, the β-CD hydrophilic exterior preferably attaches with electrophilic functional groups, such as carboxyls and amines, through electrostatic interactions.23,24 Therefore, β-CD is a useful probe to evaluate the extent of functional groups on a substrate surface. Dopamine (DA) was chosen as a guest molecule to bind selectively with β-CD to form stable host-guest inclusion complexes. DA is not only an important catecholamine neurotransmitter in the human brain,25 but is also used as an intravenous medication used to increase heart rate and blood pressure.26 Therefore, trace level determination of DA in vivo/vitro is important in common medical practice, in determining pathologic states, and in developing future diagnostic methods. Compared to other techniques, electrochemical detection of DA is attractive due to high sensitivity and ease of use.27–34
In this paper, we report for the first time a two-step electrochemical method for transforming GO into HGO using electrostatic assembly of GO film onto a 3-aminopropyltrimethoxysilane (APTMS) SAM modified glassy carbon electrode (GCE). The electrochemical approach is an effective way to control geometries and size distribution of holes. We developed a new strategy by exposing the basal plane of graphene oxide and introducing oxidative holes on it using a cyclic voltammetry-based electrochemical reduction of GO followed by the oxidation of reduced graphene oxide. The GO-, ERGO-, and HGO-modified electrodes were characterized by atomic force microscopy (AFM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, and cyclic voltammetry. In this study, DA was chosen as a probe to evaluate the electrocatalytic behavior of β-CD modified-electrode materials and to verify the redox reaction-based electrocatalytic performance of β-CD/HGO nanohybrids.
Experimental
Materials
Graphite powder (300 mesh, 99.5%) was purchased from Alfa Aesar. Sulfuric acid (93–98 w/w%, density 1.84 g cm−3 at 20 °C) was obtained from BDH Chemicals. Other chemicals, KMnO4, H2O2 (30%), NaNO3, KCl, 3-aminopropyltrimethoxysilane, K3[Fe(CN)6], K4[Fe(CN)6], Na2HPO4·7H2O, KH2PO4, β-cyclodextrin hydrate and dopamine hydrochloride, were purchased from Sigma-Aldrich (USA). All chemicals were analytical grade and were used as received. All stock solutions were prepared using deionized water (18 MΩ cm) and deoxygenated by purging with ultrahigh pure Ar before starting experiments.Preparation of GO sheets
GO was prepared by acid oxidation of graphite powder according to the popular modified Hummer's method.6 Briefly, 2 g of 300 mesh graphite powder, 2 g of NaNO3, and 96 mL of concentrated H2SO4 were mixed at 0 °C. A magnetic stirrer (stirring speed, 100 rpm) was continuously used during the entire procedure. Then, 12 g KMnO4 was slowly added to the above mixture while maintaining the temperature at 0 °C. The resultant solution was held at 0 °C for 90 min and then heated at 35 °C for 2 h. To complete the reaction, 200 mL of distilled water was added followed by 10 mL of H2O2 (30%) with continued stirring for 10 min to obtain a graphite oxide suspension. Subsequently, the suspension was subjected to high-speed centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, repeatedly washed with distilled water until the pH was ∼7, and then mild sonication (10 min, 80 W) was used to exfoliate the graphite oxide to obtain a GO suspension. Finally, GO was collected via centrifugation for 5 min at 5000 rpm and then dried in a vacuum oven for 24 h at 90 °C to completely remove the water.
000 rpm for 10 min, repeatedly washed with distilled water until the pH was ∼7, and then mild sonication (10 min, 80 W) was used to exfoliate the graphite oxide to obtain a GO suspension. Finally, GO was collected via centrifugation for 5 min at 5000 rpm and then dried in a vacuum oven for 24 h at 90 °C to completely remove the water.
Fabrication of GO/APTMS films on GCE
The GO films were fabricated by a self-assembly method as reported by Zhijuan et al.35 and Chauhan et al.36 Briefly, GO nanosheets were adsorbed on the surface of 3-aminopropyltrimethoxysilane (APTMS)-modified glassy carbon electrodes (APTMS/GCE). As shown in Fig. S1 (ESI†), GCE was preconditioned in 0.5 M H2SO4 by cycling ten times at potential window −1.0 to +1.0 V (vs. Ag/AgCl, sat. KCl) at a scan rate of 100 mV s−1. Then the activated GCE was immersed in 1% APTMS anhydrous toluene solution for 15 h allowing the assembly of the APTMS molecules onto the activated GCE surface and reaction via the C–O–Si bond.36 After being washed with copious amounts of anhydrous toluene and dried in ambient air, this APTMS-modified electrode was dipped in a GO aqueous solution (1 mg mL−1) for 4 h. Thus GO was self-assembled onto the APTMS-modified GC electrode via electrostatic interaction between the positively charged amine groups on the SAM terminus and negatively charged carboxyl groups present on the GO. This electrode, termed as GO/APTMS/GC electrode, was electrochemically reduced followed by oxidation of the reduced graphene oxide in 0.5 M H2SO4. The resulting electrochemically reduced and oxidized electrodes are termed as ERGO/APTMS/GC and HGO/APTMS/GC, respectively. Fig. 1 presents a schematic representation for the modification of GCE.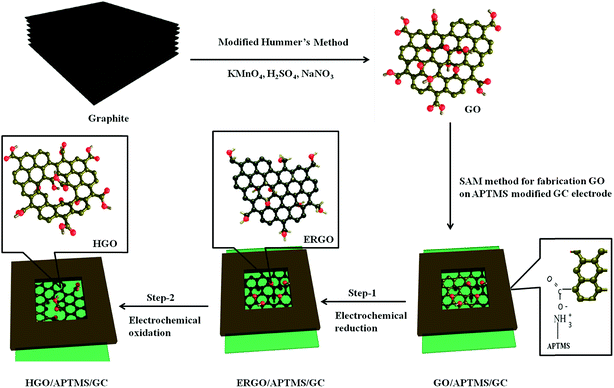 | ||
| Fig. 1 Schematic of a 2-step electrochemical process for production of holey graphene oxide (HGO) from exfoliated graphene oxide (GO). | ||
Preparation of the β-CD modified graphene electrodes
β-CD modified electrodes were prepared according to the process reported elsewhere.37 In brief, the deposition of a self-assembled monolayer of β-CD over the different surface-modified graphene electrodes described previously was carried out by immersion into a 1.0 mM aqueous solution of β-CD for 12 h at room temperature. Electrodes were then washed using ultrapure water to remove the non-adsorbed β-CD molecules on the electrode surface. The resulting modified electrodes are termed as β-CD-GO/APTMS/GC, β-CD-ERGO/APTMS/GC and β-CD-HGO/APTMS/GC.Characterization
The electrochemical measurements were carried out with a CHI 440 electrochemical analyzer (CH Instruments, Austin, TX, USA), using a conventional three-electrode system. The glassy carbon electrode (GCE, 3 mm diameter), Ag/AgCl/saturated KCl (Model CH111, CH Instruments, Austin, TX, USA), and a platinum wire were used as working, reference, and counter electrodes respectively. All experimental solutions were deoxygenated for 20 min using high purity argon and maintained under argon atmosphere during measurements. FT-IR spectra were collected on a Thermo Nicolet Model Nexus 870 FT-IR spectrometer over a 400 to 4500 cm−1 wavenumber range with DTGS or MCT detector. Raman spectroscopic measurements were carried out using a continuous wavelength DPSS laser from laser glow technology (LUD-670) operating at 670 nm as an excitation light source. An InPhotonics 670 nm Raman fiber optic probe was used for excitation and data collection. It is a combination of 90 μm excitation fiber and 200 μm collection fiber with filtering and steering micro-optics. A miniaturized QE65000 scientific-grade spectrometer from Ocean Optics was used as the Raman detector, with a 220–3600 cm−1 spectral response range. It was equipped with TE-cooled 2048 pixel CCD and interfaced to computer via a USB port. X-ray photoelectron spectroscopy (XPS) was performed with a Mg Kα X-ray source (300 W, 15 kV) and a 45° take-off angle. An Omni Focus II small-area lens and a PHI 10-360 spherical capacitor energy analyzer were used for data acquisition. Survey and high-resolution spectra were collected using a minimum of 10 scans with a 26.95 eV pass energy across the 1100 to 0 eV range and a minimum of 15 scans with a 23.5 eV pass energy with a 0.1 eV step size, respectively. For XPS analysis, electrode materials were deposited onto UV/O-treated, Au-coated Si wafers and allowed to dry inside a ventilated hood for 24 h. Spectra from a minimum of three areas on each sample were used for XPS analyses. Gaussian peak fitting was performed using CasaXPS software v.2.2.88, and average results are reported. Transmission electron microscopy (TEM) was performed using a JEOL-2100F transmission electron microscope operated at 100 kV. For TEM analysis, sample droplets were placed on Cu grids (Electron Microscopy Science) and the solvent allowed to evaporate at ambient temperature inside a ventilated hood. AFM data was collected with a Dimension Icon operated in ScanAsyst and tapping modes. Bruker's PeakForce KPFM probes with a 300 kHz resonance frequency, 42 N m−1 spring constant, and 2 nm sharp tip were used for all AFM measurements. For AFM imaging, the GC electrode samples were dispersed into water by ultrasonication for 20 min. Then ∼10 μL of the dispersed sample was drop casted onto freshly cleaned Si wafers, spin-coated at 1000 rpm and allowed to dry under ambient conditions for 30 min prior to imaging. SEM images were taken using a ZEISS Supra 40 VP scanning electron microscope (SEM) on samples ‘as formed’ on the GC electrodes. Note that the data for multiple measurements (n = 3) on five independently prepared electrode samples indicates that process was very repeatable, and the electrode modifications was relatively homogeneous.Results and discussion
Investigation of two-step electrochemical treatment of GO/APTMS/GC electrode
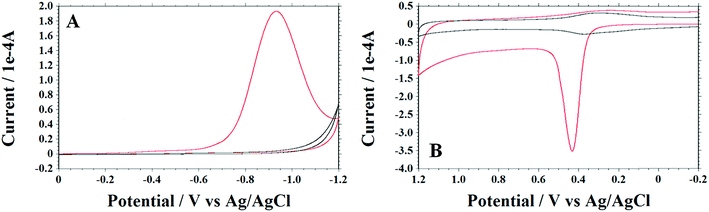
Fig. 1 represents the fabrication of GO, followed by a two-step electrochemical process for preparing holey graphene oxide (HGO). For the first step (step-1), electrochemical reduction of the obtained GO/APTMS/GC electrode was conducted by cyclic voltammetry (CV) over the potential range from 0.0 to −1.2 V vs. Ag/AgCl (3 M KCl sat.) in 0.5 M H2SO4 at scan rate of 50 mV s−1 (Fig. 2A). A large cathodic current peak at −1.0 V appeared during the first cycle which might be due to the reduction of oxygen-containing functional groups (epoxy, carboxyl, carbonyl and hydroxyl) on the GO surface. During the second cycle, the reduction peak current almost vanished, but the reduction current at switching potential at −1.2 V increased sharply for several cycles (7 cycles, see Fig. S2, ESI†). With further cycling, this reduction current decreased gradually, and stabilized after 30 cycles. The final cycle is shown by the black line in Fig. 2A, and indicates a complete reduction of oxygen-containing functional groups on GO surface.For the second step (step-2) of the treatment, just after finishing the cathodic cycle, an anodic scan was run starting over the potential window from −0.2 to +1.2 V vs. Ag/AgCl at scan rate 50 mV s−1 in 0.5 M H2SO4 (Fig. 2B). In the first cycle, the CV shows a huge oxidation peak that appeared at +0.4 V potential in the forward scan, and a weak peak appeared at +0.2 V in the backward scan. This result indicates the introduction of oxygen-containing functional groups on the graphene surface that could not be reduced in reverse scan within the applied potential range. In the second cycle, the redox peak was completely different; the intensity of the oxidation peak was decreased and shifted to around +0.35 V in the forward scan, but it was not shifted in the reversed scan. With further cycling, intensity of the oxidation and the reduction peak at +0.35 V and +0.2 V, respectively, increased gradually. The oxidation peak at +0.35 V indicates the introduction of a new oxygen-containing functional group, different than the one related to the +0.4 V peak seen in the first cycle. Moreover, this functional group at +0.35 V could be reduced at +0.2 V. On the other hand, in the first cycle, the reduced peak appearance at +0.2 V suggests that the introduction of the functional group at +0.35 V may be dominated by the one at +0.4 V. In addition, we observed that the oxidation edge current was decreasing, and then remained constant after 15 cycles (the final cycle is in black line, Fig. 2B). This result suggests that from the 2nd to the 15th cycle, a redox active oxygen containing functional group, as for example, the quinone-like groups can be reduced to hydroquinone and oxidized back reversibly. Moreover, the redox active oxygen was gradually attached on the graphene surface, reaching the maximum level.38
The electrochemical phenomena displayed by these modified graphene surfaces suggest the following:
(1) The self-assembly method favors the formation of a single or very few layers of graphene oxide. Oxygen-containing functional groups, especially carboxyl groups on the edge carbon, were mostly occupied by amine functionalities attached to the APTMS linker.39 Therefore, only basal carbon on graphene sheets were available for both electrochemical reduction and oxidation.
(2) In the first step, the oxygen surface functional groups participated in a reduction as demonstrated by a huge reduction peak at the first scan (Fig. 2A, red line). After successive reduction scans, the graphene surface was uniformly functionalized mostly by hydroxyl groups, as further discussed in the XPS and FTIR sections, resulting from the transformation of oxygen functional moieties present in carboxyl, carbonyl, and epoxide groups. As reported elsewhere,40 epoxy groups which are found predominately at the basal plane, are more stable than carboxyl and carbonyl groups. Therefore, breaking the epoxy bond (C–O) groups possibly requires additional cycle runs. It is suggested that the 1st reduction cycle favors the conversion of carboxyl and carbonyl to the corresponding hydroxyl groups having lower energy barrier, and then cycles 2 to 30 contribute higher energy to meet the large energy barrier conversion of epoxy to hydroxyl groups. This phenomenon might vary based on the quality of graphene oxide in terms of their defects such as impurities (carbonaceous and metallic), thickness, and homogeneity of the coating. Moreover, the resultant single layer (or few layers) of reduced graphene oxide might have distorted benzene rings after losing the oxygen containing functional group on ring carbon.8 Indeed, wavy-silk GO was transformed to a surface of high density wrinkles, folds and rolled edges (SEM image of reduced GO, see Fig. S3, ESI†). In addition, the edge-plane-like defective sites were created on the basal plane of reduced graphene sheets, making the surface more sensitive to the next attack by anodic oxidation.
(3) In the second step, the vulnerable single layer (or few layers) of reduced graphene oxide was exposed to the surrounding active oxygen (O2 + 2H+ + 2e → H2O2 created during cathodic scan and SO3H− from H2SO4) at the close vicinity of the electrode surface.41–43 These active oxygen species can possibly break the distorted C–C single bond of the benzene ring (where each carbon is bonded with either hydroxyl or oxygen of another epoxy group) on the graphene sheet creating holes on the graphene oxide surface. This observation is also consistent with previously reported epoxy chain-induced unzipping of carbon rings.44–46 It is also suggested that once a hole has been initiated, it will continue until relieved by the benzene ring bond-angle strain, adjusting the regular energy distribution across the planar graphene sheets. GO after the electrochemical process was characterized using morphologic and spectroscopic characterizations, as described next.
Surface morphology characterization
The surface morphology and structure of exfoliated GO, ERGO and HGO were investigated using atomic force microscopy (AFM), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Fig. 3 shows the atomic force microscopy of the exfoliated GO and HGO. As shown in Fig. 3A, the exfoliated GO sheets were flat, nonporous and straight edges with an average thickness of about 1.6 nm (see Fig. S4, ESI†). On the other hand, the thickness of the ERGO and HGO was about 2.7 and 3.6 nm, respectively (Fig. 3B and S4, ESI†). In this case, the thickness was increased due to the APTMS linker attached on the backside of ERGO and HGO sheets. After electrochemical reduction of GO, the resultant surface became smooth compared to that of GO. This difference was clearly observed using the height profiles along the cross section lines of the corresponding surfaces (Fig. S4, ESI†). More importantly, the HGO edges are no longer straight and pores, with diameters ranging from a few nanometers to several hundred nanometers, are randomly distributed across the entire sheets due to oxidation of the reduced form of graphene oxide. | ||
| Fig. 3 AFM topography images collected in tapping mode for exfoliated GO (A) and HGO (B). Both samples, GO and HGO, were prepared by drop-casting dilute dispersions onto clean silicon wafers. | ||
Fig. 4A–D contrast the resulting surface features of GO following electrochemical transformation to HGO. According to the SEM and TEM observations, the wavy-silk GO was changed into HGO displaying a flat graphene surface with holes. Fig. 4E and F show the nanohybrids of β-CD with GO and HGO respectively. In TEM image of β-CD/GO (Fig. 4E), we observed that β-CD was selectively anchored with edge carbon functional group mostly carboxyl (hydrophilic character) rather than that of basal carbon mostly hydroxyl group (hydrophobic character) of graphene sheet. Conversely, in HGO (Fig. 4F), β-CD was randomly immobilized on the graphene sheet due to increasing edge associated hydrophilic functional group (carboxyl) across the entire sheet.
 | ||
| Fig. 4 SEM images of GO/APTMS (A) and HGO/APTMS (B) on GC electrode. TEM images of exfoliated GO (C), HGO (D), and the characteristic features of immobilized β-CD on GO (E) and HGO (F), respectively. | ||
Spectroscopic characterization
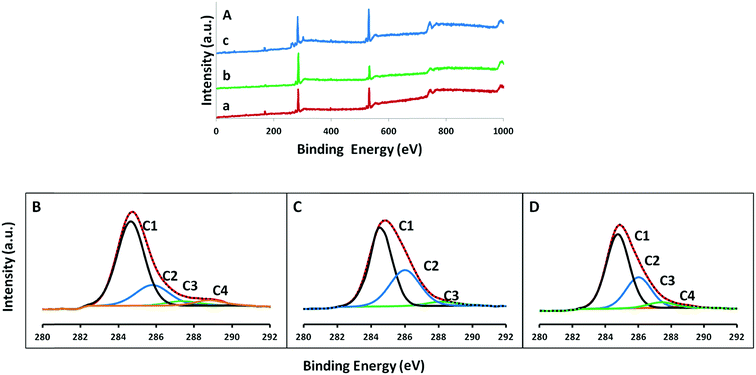
In order to confirm the oxidative holes on graphene plane and changes in the functional groups attached to the different graphene surfaces (GO-, ERGO- and HGO-modified substrate), we carried out X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR), and Raman spectroscopy analyses. Fig. 5A shows the XPS survey spectra for the GO/APTMS/GC, ERGO/APTMS/GC and HGO/APTMS/GC substrates. All substrates show two major peaks, at 285.0 and 531.6 eV, that correspond to C 1s and O 1s, respectively. Other minor peaks at 398, 154, and 104 eV were assigned to N 1s, Si 2s and Si 2p respectively.47 Interestingly, for exfoliated GO only, N and Si peaks were not observed (Fig. S5, ESI†). This result suggests the successful attachment of GO on GC electrode through the amine functionality of the APTMS linker. Moreover, the C/O ratio of both GO (with and without APTMS) were similar, suggesting GO/APTMS could be considered as a representative of GO material for comparing ERGO and HGO-substrate materials (see Table 1). After electrochemical reduction of GO-modified substrate, the C/O intensity ratio (Fig. 5A, curve b) increased from 2.09 to 4.14 indicating the removal of surface oxygen functional groups from GO.12 Furthermore, after electrochemical oxidation of the reduced form of GO, the intensity ratio C/O was decreased from 4.14 to 1.84 indicating the introduction of oxygen functional group on the graphene surface. These results are consistent with previous reports, and confirm the overall two-step electrochemical reduction and oxidation treatment.48| Sample | C 1s (%) | O 1s (%) | C/O |
|---|---|---|---|
| Exfoliated GO only | 68.2 | 31.8 | 2.14 |
| GO/APTMS/GCE | 67.6 | 32.4 | 2.09 |
| ERGO/APTMS/GCE | 80.6 | 19.4 | 4.14 |
| HGO/APTMS/GCE | 64.9 | 35.1 | 1.84 |
Additionally, Table 1 shows the atomic percentage of carbon and oxygen in different substrate materials. Different types of carbon and oxygen functional groups were also confirmed by C 1s and O 1s XPS high resolution scans. The high resolution C 1s spectrum of GO substrate in Fig. 5B shows four peaks at 285.0, 285.8, 287.4 and 288.8 eV, corresponding to C–C sp2 carbon, C–O bond, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and HO–C
O bond and HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, respectively.49–51 As is well-known, the binding energy of 287.4 eV for C
O bond, respectively.49–51 As is well-known, the binding energy of 287.4 eV for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is similar to that of C–N, and hence a separate C–N peak was not observed in all substrate spectra.52,53 After electrochemical reduction, the C 1s spectrum of ERGO/APTMS/GC substrate in Fig. 5C shows three peaks at 285.0, 286.0, and 288.2 eV corresponding to the C–C sp2 carbon, C–O bond and C
O is similar to that of C–N, and hence a separate C–N peak was not observed in all substrate spectra.52,53 After electrochemical reduction, the C 1s spectrum of ERGO/APTMS/GC substrate in Fig. 5C shows three peaks at 285.0, 286.0, and 288.2 eV corresponding to the C–C sp2 carbon, C–O bond and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond respectively. In this spectrum, the intensities of oxygen-containing functional groups were lower compared to GO-modified substrate. Conversely, four C 1s (sp2 carbon, C–O, C
O bond respectively. In this spectrum, the intensities of oxygen-containing functional groups were lower compared to GO-modified substrate. Conversely, four C 1s (sp2 carbon, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and HO–C
O and HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) peaks with high intensity of different oxygen functional groups were re-observed at the C 1s spectrum of HGO/APTMS/GO substrate (Fig. 5D). High resolution XPS spectra of O 1s in Fig. 6 (C–O at 531.8 eV, C
O) peaks with high intensity of different oxygen functional groups were re-observed at the C 1s spectrum of HGO/APTMS/GO substrate (Fig. 5D). High resolution XPS spectra of O 1s in Fig. 6 (C–O at 531.8 eV, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 533.8 eV and HO–C
O at 533.8 eV and HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 534.8 eV) also follows this trends.54 In particular, after electrochemical oxidation, the intensities of oxygen functional groups HO–C
O at 534.8 eV) also follows this trends.54 In particular, after electrochemical oxidation, the intensities of oxygen functional groups HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O were obviously increased as compared to that of GO-modified substrate, and the overall oxygen content was found to increase to 16.0 at.%. This result indicates that higher edge carbon associated with holes on graphene sheet facilitates the formation of higher carboxyl functional groups at HGO-modified substrate.
O were obviously increased as compared to that of GO-modified substrate, and the overall oxygen content was found to increase to 16.0 at.%. This result indicates that higher edge carbon associated with holes on graphene sheet facilitates the formation of higher carboxyl functional groups at HGO-modified substrate.
Further analysis using the FTIR transmittance spectra (KBr) were performed on (a) GO-, (b) ERGO-, and (c) HGO-substrates (Fig. 7) to carefully study the oxidation level and chemical functionalities of different graphene surfaces. The spectrum of HGO illustrates high absorption of O–H group in the regions of O–H (500–900 cm−1 and 3000–3700 cm−1) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O modes (1700–1750 cm−1) and lower absorption in the regions of C
O modes (1700–1750 cm−1) and lower absorption in the regions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H (800–1000 cm−1) and C–O modes (1000–1250 cm−1). When GO was electrochemically reduced, the C
C–H (800–1000 cm−1) and C–O modes (1000–1250 cm−1). When GO was electrochemically reduced, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration band almost disappeared, the broad O–H and C–O stretching bands remained, and C
O vibration band almost disappeared, the broad O–H and C–O stretching bands remained, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H band was newly appeared. It suggests the optimal reduction of GO surface with some degree restoration of C
C–H band was newly appeared. It suggests the optimal reduction of GO surface with some degree restoration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonding result in a high pure graphene surface. While ERGO was re-oxidized electrochemically, the spectrum of HGO exhibited a stronger IR absorption by C
C bonding result in a high pure graphene surface. While ERGO was re-oxidized electrochemically, the spectrum of HGO exhibited a stronger IR absorption by C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O modes compared to GO. The most striking contrast was observed in the HGO spectrum with the large growth of two broad –OH absorption bands in regions 500–1000 and 3000–3700 cm−1 compared to both RGO and GO spectra. This observation implies that electrochemical oxidation of ERGO alleviates a build-up of O–H groups on RGO sheets possibly providing a pathway for further oxidation, resulting in the formation of holes on graphene sheets.
O and C–O modes compared to GO. The most striking contrast was observed in the HGO spectrum with the large growth of two broad –OH absorption bands in regions 500–1000 and 3000–3700 cm−1 compared to both RGO and GO spectra. This observation implies that electrochemical oxidation of ERGO alleviates a build-up of O–H groups on RGO sheets possibly providing a pathway for further oxidation, resulting in the formation of holes on graphene sheets.
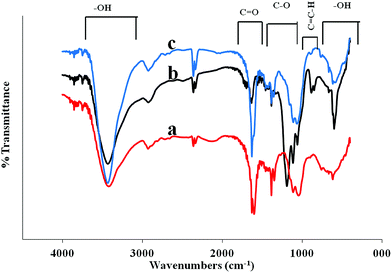 | ||
| Fig. 7 FTIR transmittance spectra of GO/APTMS/GC (a), ERGO/APTMS/GC (b) and HGO/APTMS/GC (c) substrates supporting each reaction step. | ||
Lastly, we employed Raman spectroscopy as a widely used nondestructive technique to characterize ordered and disordered crystal structures of carbon nanomaterials. We used spectroscopic probe to investigate the structural changes of graphene carbon networks due to reduction/oxidation of surface functional groups during the electrochemical process. The Raman spectrum of the pristine graphite has been reported to display a strong G band at 1579 cm−1, a weak D band at 1360 cm−1, and a middle 2D and D + D′ bands at 2700 cm−1 and 2940 cm−1 respectively.55 In Fig. 8, the D and G bands of GO/APTMS/GC (curve b) substrate spectrum were broadened and shifted at positions 1365 cm−1 and 1607 cm−1, respectively, due to the severe defects resulting from oxidative exfoliation of pristine graphite (curve a). After completing reduction cycles, in ERGO/APTMS/GC substrate, a clear spectrum (Fig. 8, curve c) with sharp D and G bands was observed at 1338 cm−1 and 1620 cm−1, respectively. It suggests that the order of graphene carbon in-plane sp2 domain is partly recovered during the electrochemical reduction that is consistent with previous reports.56 The intensity ratio of D and G bands (ID/IG) of carbon materials is usually assigned to demonstrate the extent of surface modification or defects arising from distortion, vacancies and edges of hexagonal network of carbon atoms.57 As shown in Fig. 8, the intensity ratio (ID/IG = 1.23) for ERGO-modified substrate is higher than that of GO (ID/IG = 0.91). This observation is attributed to the increased defect concentration present in ERGO relative to that in GO. Moreover, in ERGO-substrate, a broad and weak 2D and D + D′ band at 2652 cm−1 and 2928 cm−1, respectively, was observed due to the hydrogenation of graphene surface.58 Then, after running anodic cycles the intensity of both D and G bands was decreased comparing to that in ERGO-modified substrate but similar to the peaks observed for GO-modified substrate. But in this case, the intensity ratio (ID/IG = 1.01) of HGO/APTMS/GC substrate is higher than that in GO-modified substrate. In addition, there is a significant change in 2D and D + D′ bands. The weak and broadened 2D and D + D′ bands of ERGO-modified substrate were completely disappeared in HGO-modified substrate spectrum. The results are consistent with prior literature findings10,59 suggesting that the decreased intensity ratio and significant change in 2D and D + D′ bands of HGO comparing to ERGO-substrate are due to the introduction of oxygenated functionalities that disrupt the planar sp2 structure, resulting in cracks or holes on the graphene surface. The peak assignments for Raman spectra and XPS are summarized in Table S1 (ESI†).
 | ||
| Fig. 8 Raman spectra of pristine graphite (a), GO/APTMS/GC (b), ERGO/APTMS/GC (c), and HGO/APTMS/GC (d) substrates. | ||
Electrochemical characterization
We employed cyclic voltammetry (CV) of [Fe(CN)6]3−/4− redox probe to investigate the intrinsic electron transfer properties of the obtained holey graphene oxide (HGO) and compared its electrochemical behavior with GO- and ERGO-modified electrodes. Fig. 9A shows the electrochemical responses of bare GC, GO/APTMS/GC, ERGO/APTMS/GC and HGO/APTMS/GC electrodes to argon saturated 1.0 mM K3[Fe(CN)6]/K4[Fe(CN)6] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) in 1.0 M KCl electrolyte. All the electrodes show well-defined peaks in the forward and reverse scans due to the oxidation and reduction of [Fe(CN)]3−/4− redox couple. In all cases, the anodic (Ipa) and cathodic (Ipc) peak currents are varied linearly with respect to the square root of scan rate (v1/2), indicating that the electrode kinetics are controlled by mass transport (semi-infinite linear diffusion) of the reactant (Fig. 9B). This criteria of electrode surface is followed by the Randles–Sevcik equation (eqn (1)),60
1 molar ratio) in 1.0 M KCl electrolyte. All the electrodes show well-defined peaks in the forward and reverse scans due to the oxidation and reduction of [Fe(CN)]3−/4− redox couple. In all cases, the anodic (Ipa) and cathodic (Ipc) peak currents are varied linearly with respect to the square root of scan rate (v1/2), indicating that the electrode kinetics are controlled by mass transport (semi-infinite linear diffusion) of the reactant (Fig. 9B). This criteria of electrode surface is followed by the Randles–Sevcik equation (eqn (1)),60| Ip = 2.687 × 105ACn3/2(Dν)1/2 | (1) |
Electrochemical catalysis of dopamine (DA) on different electrodes modified by β-cyclodextrin (β-CD)
To further evaluate the practical application of HGO in the field of electrochemical sensors, we also examined the nanocomposite of β-cyclodextrin (β-CD) with HGO modified electrode, and its electrocatalytic activity toward dopamine (DA) sensing. Since a large number of active sites containing polar groups (mostly carboxyl) have been developed on HGO surface, large amounts of β-CD could be immobilized on the surface due to the hydrophilic interactions with the exterior hydroxyl groups of β-CD. Moreover, the prepared β-CD modified HGO electrode could serve as a sensitive and selective platform by forming an inclusion complex with the dopamine molecule via hydrogen bonding interactions, which might increase the response of the sensor.66 Fig. 10 shows cyclic voltammograms (CVs, 1st cycle) of DA (1.0 mM) in phosphate buffer solutions (PBS 0.1 M, pH 7.40) at scan rate 50 mV s−1 on β-CD-GO/APTMS/GC (curve a), β-CD-ERGO/APTMS/GC (curve b), HGO/APTMS/GC (curve c), bare GCE (curve d), and β-CD-HGO/APTMS/GC (curve e).It is well known that there are four DA oxidation/reduction peaks that correspond to the reactions shown in Scheme 1.67,68
The most obvious observation from Fig. 10 is that the highest two pairs of oxidation/reduction peak currents corresponding to above four reactions of DA were at β-CD-HGO/APTMS/GC among all other modified electrodes, indicating a favorable catalytic activity of β-CD/HGO towards the oxidation/reduction of this compound. It was also observed that the double layer capacitance at β-CD/HGO is much higher when compared to only HGO modified electrode (curve c: dotted line), which means that β-CD provides a much higher electrochemical active area for enhanced electrochemical response toward DA. As shown in Fig. 10e, the voltammogram displays anodic peak at 0.182 V vs. Ag/AgCl and a cathodic peak at 0.142 V vs. Ag/AgCl corresponding to the oxidation of DA to o-dopaminoquinone (DAQ) and the reduction of DAQ back to DA, respectively (eqn (1)). At pH 7.4, DAQ undergoes intramolecular cyclization through deprotonation of the amino group to form leucodopaminochrome (LDAC) (eqn (2)). Thus, a redox pair with a cathodic peak at −0.273 V vs. Ag/AgCl and an anodic peak at −0.229 V vs. Ag/AgCl are assigned to the redox couple LDAC/dopaminochrome (DAC) (eqn (3)). We need to mention that the anodic peak at −0.229 was not observed as it was not yet formed in the first cycle (Fig. S6, ESI†).
On the other hand, for the β-CD-GO/APTMS/GC, β-CD-ERGO/APTMS/GC, HGO/APTMS/GC, and bare GC electrodes, the CV scan displays an anodic peak at 0.304, 0.184, 0.204, 0.213 V and a cathodic peak at 0.066, 0.128, 0.119, 0.067 V assigning to the DA/DAQ redox couple shown in Fig. 10a–d respectively. Both (anodic and cathodic) peak currents for β-CD/ERGO, only HGO, and β-CD/GO nanohybrid electrodes were much smaller than that of the β-CD/HGO electrode. Moreover, β-CD/GO showed anodic peak potential shift toward more positive as compared to the peak of β-CD/ERGO and only HGO electrode. In addition, the oxidation peak current of DA was enhanced at bare GC electrode comparing to β-CD/ERGO, only HGO and β-CD/GO. Besides, the DA/DAQ redox peak difference ΔEp for β-CD/GO, β-CD/ERGO, only HGO and bare GC were 0.259, 0.09, 0.082 and 0.147 V, respectively, while the β-CD/HGO electrode was 0.04 V. All observations suggest that β-CD was not properly anchored due to the hydrophobic surface interaction with ERGO/APTMS/GC and the limited anchoring through hydrophilic interaction along the edges (as shown in TEM image Fig. 4E) of GO/APTMS modified GC electrode. In contrast, HGO favors β-CD molecules immobilized on its surface providing high density of active sites resulting enhanced electrocatalytic activity toward DA when compared to all other electrodes.
Amperometric response of DA at the β-CD-HGO/APTMS/GC electrode
In order to determine the linear range and sensitivity of the prepared β-CD-HGO/APTMS/GC electrodes for DA detection, amperometric responses are displayed in Fig. 11A. The response currents were monitored at an applied potential fixed at 0.200 V after successive additions of DA into 0.1 M PBS (pH 7.40). Fig. 11C shows the linear response of the oxidative current density against the corresponding DA concentration for this electrode. The linear regression equation was I (μA cm−2) = 0.0044c (μM) + 0.1204, with a correlation coefficient of 0.9907. From the slope of 0.0044 μA μM−1.cm2, the detection limit was estimated to 7.6 nM (S/N = 3) using the following method: limit of detection, LOD = SDbackground/S, where S = slop or sensitivity, SD = standard deviation. The linear range of the β-CD-HGO/APTMS/GC was 0.1–800 μM while the sensitivity to the oxidation of DA was 4.4 nA μM−1 cm−2. The comparison of β-CD-HGO/APTMS/GC with other DA sensor previously reported is demonstrated in Table 2. This electrode shows better linearity range and detection limit than other modified electrodes.| Modified electrode | Linear range (μM) | Detection limit (μM) | Reference |
|---|---|---|---|
| a Polyamide 6/poly-allylamine hydrochloride.b Carbon nanofibers.c Poly(3,4-ethylenedioxythiophene)/poly(N-methylpyrrole). | |||
| PA6/PAHa_MWCNTs/ITO | 1.0–70 | 0.15 | 28 |
| Ag–Pt/p-CNFsb/GCE | 10.0–500 | 0.11 | 31 |
| PEDOT/PNMPyc/AuNPs/GCE | 1.0–100 | 2.0–3.0 | 29 |
| Nano Pd–Au particles/GCE | 0.5–6.95 | 0.5 | 32 |
| Reduced graphene oxide/GCE | 5.0–200 | 2.0 | 34 |
| MWCNT/CPE | 20–50 | 1.07 | 30 |
| Pt@Au/MWNTs/GCE | 10–120 | 0.08 | 35 |
| PANI-GO/GCE | 2.0–18 | 0.50 | 33 |
| β-CD-HGO/APTMS/GC | 0.1–800 | 7.6 nM | This study |
Stability, reproducibility, and anti-interference property of the β-CD-HGO/APTMS/GC electrode
Since the electrode preparation method is simple and rapid, it is not so important for the electrode to be stable over a long period of time. However, this β-CD-HGO/APTMS/GC electrode results in higher stability for amperometric measurement at a constant potential. Fig. 11B displays the stability of the response without DA in 0.1 M PBS (pH 7.40) at an applied potential of 0.200 V. The response remains stable throughout 1 hour. We also evaluated the reproducibility by measuring amperometric (Eap = 0.2) responses of the five separately prepared β-CD-HGO/APTMS/GC electrodes in pH 7.4 with sequential addition of 0.1 mM of DA in 0.1 M PBS. The test result was shown in Table S3 (ESI†). The relative standard deviation (R.S.D) was 6.1% suggesting the high reproducibility of the preparation method of this modified electrode. Moreover, Fig. 11D shows the amperometric response of β-CD-HGO/APTMS/GC investigated in pH 7.4 PBS with sequential additions of 0.05 mM DA and potential interferents including uric acid (UA) and ascorbic acid (AA) of 0.1 mM for each addition. A well-defined DA response was obtained and insignificant responses were observed for interfering species. The observation suggests that this electrode shows good selectivity for DA detection.Conclusions
In summary, we have developed an unprecedented method of generating holes on basal plane of graphene oxide (GO) by a complete electrochemical method with reduction followed by oxidation of GO. The current limitations of chemically synthesized holey graphene oxide (HGO), due to over-oxidation and defects of the graphene surface, could be eliminated by using controlled-potential-step experiments as demonstrated in this study. The as-synthesized HGO exhibited high active surface area, high electron transfer, and good chemical stability, as well as being an attractive platform for electrode modification. Particularly, the β-CD-HGO nanohybrid produced using electrochemically-generated HGO showed much higher electrocatalytic activity for the oxidation of dopamine than that of β-CD-GO and β-CD-ERGO. This finding implies that the presence of oxygen functionalities in conjugation with additional edges associated with holes in HGO may contribute to the SAM decoration of other nanomaterials or bio-molecules by a defect-mediated nucleation, and lead to formation of an enhanced electrochemical platform for constructing efficient biosensing, biomedical, energy conversion systems. This trait could open a new horizon in nanoelectronics, supercapacitors, solar cells and sensor devices.Acknowledgements
The authors would like to offer a special thanks to Dr Hiroyasu Tachikawa and Dr Paresh Chandra Ray for access to electrochemical workstation and Raman spectroscopy, and also to acknowledge financial support from the National Science Foundation (EPS-362492-190200-01; EPS-0903787; HRD 0833178; RCMI-8G12MD007581).References
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- M. Pumera, Chem. Soc. Rev., 2010, 39, 4146–4157 RSC.
- J. Shen, Y. Hu, C. Li, C. Qin and M. Ye, Small, 2009, 5, 82–85 CrossRef CAS PubMed.
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Chem. Rev., 2012, 112, 6156–6214 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- A. Lerf, H. He, M. Forster and J. Klinowski, J. Phys. Chem. B, 1998, 102, 4477–4482 CrossRef CAS.
- S. Mao, H. Pu and J. Chen, RSC Adv., 2012, 2, 2643–2662 RSC.
- J. G. Radich and P. V. Kamat, ACS Nano, 2013, 7, 5546–5557 CrossRef CAS PubMed.
- G. P. Kotchey, B. L. Allen, H. Vedala, N. Yanamala, A. A. Kapralov, Y. Y. Tyurina, J. Klein-Seetharaman, V. E. Kagan and A. Star, ACS Nano, 2011, 5, 2098–2108 CrossRef CAS PubMed.
- Y. Lin, K. A. Watson, J.-W. Kim, D. W. Baggett, D. C. Working and J. W. Connell, Nanoscale, 2013, 5, 7814–7824 RSC.
- H.-L. Guo, X.-F. Wang, Q.-Y. Qian, F.-B. Wang and X.-H. Xia, ACS Nano, 2009, 3, 2653–2659 CrossRef CAS PubMed.
- Y. Shao, J. Wang, M. Engelhard, C. Wang and Y. Lin, J. Mater. Chem., 2010, 20, 743–748 RSC.
- D. B. Shinde, J. Debgupta, A. Kushwaha, M. Aslam and V. K. Pillai, J. Am. Chem. Soc., 2011, 133, 4168–4171 CrossRef CAS PubMed.
- M. Zhou, Y. Wang, Y. Zhai, J. Zhai, W. Ren, F. Wang and S. Dong, Chem.–Eur. J., 2009, 15, 6116–6120 CrossRef CAS PubMed.
- X. Zhou, X. Huang, X. Qi, S. Wu, C. Xue, F. Y. C. Boey, Q. Yan, P. Chen and H. Zhang, J. Phys. Chem. C, 2009, 113, 10842–10846 CAS.
- S. Gilje, S. Han, M. Wang, K. L. Wang and R. B. Kaner, Nano Lett., 2007, 7, 3394–3398 CrossRef CAS PubMed.
- E. Shi, H. Li, L. Yang, L. Zhang, Z. Li, P. Li, Y. Shang, S. Wu, X. Li, J. Wei, K. Wang, H. Zhu, D. Wu, Y. Fang and A. Cao, Nano Lett., 2013, 13, 1776–1781 CAS.
- G. P. Keeley, A. O'Neill, M. Holzinger, S. Cosnier, J. N. Coleman and G. S. Duesberg, Phys. Chem. Chem. Phys., 2011, 13, 7747–7750 RSC.
- H. Imran, P. N. Manikandan and V. Dharuman, RSC Adv., 2015, 5, 63513–63520 RSC.
- H. G. Jeon, Y. H. Huh, S. H. Yun, K. W. Kim, S. S. Lee, J. Lim, K.-S. An and B. Park, J. Mater. Chem. C, 2014, 2, 2622–2634 RSC.
- R. Freeman, T. Finder, L. Bahshi and I. Willner, Nano Lett., 2009, 9, 2073–2076 CrossRef CAS PubMed.
- A. González-Campo, S.-H. Hsu, L. Puig, J. Huskens, D. N. Reinhoudt and A. H. Velders, J. Am. Chem. Soc., 2010, 132, 11434–11436 CrossRef PubMed.
- C. Feng, G. Lu, Y. Li and X. Huang, Langmuir, 2013, 29, 10922–10931 CrossRef CAS PubMed.
- R. M. Wightman, L. J. May and A. C. Michael, Anal. Chem., 1988, 60, 769A–793A CrossRef CAS PubMed.
- J. P. Long, S. Heintz, J. G. Cannon and J. Kim, J. Pharmacol. Exp. Ther., 1975, 192, 336–342 CAS.
- L. A. Mercante, A. Pavinatto, L. E. O. Iwaki, V. P. Scagion, V. Zucolotto, O. N. Oliveira, L. H. C. Mattoso and D. S. Correa, ACS Appl. Mater. Interfaces, 2015, 7, 4784–4790 CAS.
- G. Fabregat, E. Armelin and C. Alemán, J. Phys. Chem. B, 2014, 118, 4669–4682 CrossRef CAS PubMed.
- S. M. Ghoreishi, M. Behpour, S. Mousavi, A. Khoobi and F. S. Ghoreishi, RSC Adv., 2014, 4, 37979–37984 RSC.
- Y. Huang, Y.-E. Miao, S. Ji, W. W. Tjiu and T. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 12449–12456 CAS.
- T.-H. Tsai, S. Thiagarajan, S.-M. Chen and C.-Y. Cheng, Thin Solid Films, 2012, 520, 3054–3059 CrossRef CAS.
- P. Manivel, M. Dhakshnamoorthy, A. Balamurugan, N. Ponpandian, D. Mangalaraj and C. Viswanathan, RSC Adv., 2013, 3, 14428–14437 RSC.
- Y. Wang, Y. Li, L. Tang, J. Lu and J. Li, Electrochem. Commun., 2009, 11, 889–892 CrossRef CAS.
- Y.-C. Bai and W.-D. Zhang, Electroanalysis, 2010, 22, 237–243 CrossRef CAS.
- Z. Wang, X. Zhou, J. Zhang, F. Boey and H. Zhang, J. Phys. Chem. C, 2009, 113, 14071–14075 CAS.
- A. K. Chauhan, D. K. Aswal, S. P. Koiry, S. K. Gupta, J. V. Yakhmi, C. Sürgers, D. Guerin, S. Lenfant and D. Vuillaume, Appl. Phys. A: Mater. Sci. Process., 2008, 90, 581–589 Search PubMed.
- Y. Wei, L.-T. Kong, R. Yang, L. Wang, J.-H. Liu and X.-J. Huang, Langmuir, 2011, 27, 10295–10301 CrossRef CAS PubMed.
- S.-X. Guo, S.-F. Zhao, A. M. Bond and J. Zhang, Langmuir, 2012, 28, 5275–5285 CrossRef CAS PubMed.
- G. K. Ramesha and S. Sampath, J. Phys. Chem. C, 2009, 113, 7985–7989 CAS.
- P. V. Kumar, M. Bernardi and J. C. Grossman, ACS Nano, 2013, 7, 1638–1645 CrossRef CAS PubMed.
- P. Horsman, B. E. Conway and E. Yeager, Comprehensive Treatise of Electrochemistry: Volume 7 Kinetics and Mechanisms of Electrode Processes, Springer, US, 2012 Search PubMed.
- A. Martínez-García, A. Sánchez-Reche and J. M. Martín-Martínez, J. Adhes., 2003, 79, 525–547 CrossRef.
- W. Wei, Y. Tao, W. Lv, F.-Y. Su, L. Ke, J. Li, D.-W. Wang, B. Li, F. Kang and Q.-H. Yang, Sci. Rep., 2014, 4, 6289 CrossRef CAS PubMed.
- Z. Li, W. Zhang, Y. Luo, J. Yang and J. G. Hou, J. Am. Chem. Soc., 2009, 131, 6320–6321 CrossRef CAS PubMed.
- J.-A. Yan and M. Y. Chou, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 125403 Search PubMed.
- X. Zhiping and X. Kun, Nanotechnology, 2010, 21, 045704 CrossRef PubMed.
- M. Dubey, I. Gouzman, S. L. Bernasek and J. Schwartz, Langmuir, 2006, 22, 4649–4653 CrossRef CAS PubMed.
- M. Patel, W. Feng, K. Savaram, M. R. Khoshi, R. Huang, J. Sun, E. Rabie, C. Flach, R. Mendelsohn, E. Garfunkel and H. He, Small, 2015, 11, 3358–3368 CrossRef CAS PubMed.
- G. Sobon, J. Sotor, J. Jagiello, R. Kozinski, M. Zdrojek, M. Holdynski, P. Paletko, J. Boguslawski, L. Lipinska and K. M. Abramski, Opt. Express, 2012, 20, 19463–19473 CrossRef CAS PubMed.
- K. M. Sundberg, W. H. Smyrl, L. Atanasoska and R. Atanasoski, J. Electrochem. Soc., 1989, 136, 434–439 CrossRef CAS.
- E. S. Vasquez, I. W. Chu and K. B. Walters, Langmuir, 2014, 30, 6858–6866 CrossRef CAS PubMed.
- E. S. Vasquez, J. L. Cunningham, J. B. McMahan, C. L. Simpson and K. B. Walters, J. Mater. Chem. B, 2015, 3, 6411–6419 RSC.
- C. Malitesta, I. Losito, L. Sabbatini and P. G. Zambonin, J. Electron Spectrosc. Relat. Phenom., 1995, 76, 629–634 CrossRef CAS.
- Y. Shao, S. Zhang, M. H. Engelhard, G. Li, G. Shao, Y. Wang, J. Liu, I. A. Aksay and Y. Lin, J. Mater. Chem., 2010, 20, 7491–7496 RSC.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 14095–14107 CrossRef CAS.
- K. N. Kudin, B. Ozbas, H. C. Schniepp, R. K. Prud'homme, I. A. Aksay and R. Car, Nano Lett., 2008, 8, 36–41 CrossRef CAS PubMed.
- A. Eckmann, A. Felten, A. Mishchenko, L. Britnell, R. Krupke, K. S. Novoselov and C. Casiraghi, Nano Lett., 2012, 12, 3925–3930 CrossRef CAS PubMed.
- D. C. Elias, R. R. Nair, T. M. G. Mohiuddin, S. V. Morozov, P. Blake, M. P. Halsall, A. C. Ferrari, D. W. Boukhvalov, M. I. Katsnelson, A. K. Geim and K. S. Novoselov, Science, 2009, 323, 610–613 CrossRef CAS PubMed.
- V. C. Tung, M. J. Allen, Y. Yang and R. B. Kaner, Nat. Nanotechnol., 2009, 4, 25–29 CrossRef CAS PubMed.
- J. E. B. Randles, Trans. Faraday Soc., 1948, 44, 327–338 RSC.
- M. Halik and A. Hirsch, Adv. Mater., 2011, 23, 2689–2695 CrossRef CAS PubMed.
- H. R. Thomas, S. P. Day, W. E. Woodruff, C. Vallés, R. J. Young, I. A. Kinloch, G. W. Morley, J. V. Hanna, N. R. Wilson and J. P. Rourke, Chem. Mater., 2013, 25, 3580–3588 CrossRef CAS.
- M. Velický, D. F. Bradley, A. J. Cooper, E. W. Hill, I. A. Kinloch, A. Mishchenko, K. S. Novoselov, H. V. Patten, P. S. Toth, A. T. Valota, S. D. Worrall and R. A. W. Dryfe, ACS Nano, 2014, 8, 10089–10100 CrossRef PubMed.
- A. J. F. L. R. Bard, Electrochemical methods: fundamentals and applications, Wiley, New York, 1980 Search PubMed.
- W. Li, C. Tan, M. A. Lowe, H. D. Abruña and D. C. Ralph, ACS Nano, 2011, 5, 2264–2270 CrossRef CAS PubMed.
- Y. Wu, Z. Dou, Y. Liu, G. Lv, T. Pu and X. He, RSC Adv., 2013, 3, 12726–12734 RSC.
- M. D. Hawley, S. V. Tatawawadi, S. Piekarski and R. N. Adams, J. Am. Chem. Soc., 1967, 89, 447–450 CrossRef CAS PubMed.
- A. Ciszewski and G. Milczarek, Anal. Chem., 1999, 71, 1055–1061 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19991c |
| This journal is © The Royal Society of Chemistry 2015 |