A highly sensitive multi-catalytic sensing system for organophosphorus and organochlorine pesticides based on the peroxidase-like activity of ferric ions
Yan Xu†
a,
Tao Yu†c,
Xiao-Qiong Wua,
Jiang-Shan Shen*ab and
Hong-Wu Zhang*a
aKey Laboratory of Urban Pollutant Conversion, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen, 361021, China. E-mail: jsshen@hqu.edu.cn; hwzhang@iue.ac.cn
bCollege of Materials Science and Engineering, Huaqiao University, Xiamen, 361021, China
cState Key Laboratory of NBC Protection for Civilian, Chemical Defense Institute Research, Beijing, 102205, China
First published on 20th November 2015
Abstract
Developing rapid, efficient and highly sensitive sensing systems for organophosphorus (OPs) and organochlorine pesticides is important due to their potential damage to human health. Considering that Fe3+ ions were recently found to have much higher peroxidase-like activity than that of Fe3O4 magnetic nanoparticles, in this work, a novel and highly sensitive multi-catalytic sensing system has been successfully developed for OPs and organochlorine pesticides, on the basis of the color reaction of 3,3′,5,5′-tetramethyl benzidine (TMB) driven by Fe3+ ions, together with two enzymatic catalytic systems of acetylcholinesterase (AChE) and choline oxidase (CHO). Sub nM level limits of detection could be achieved for four tested OPs and organochlorine pesticides. Furthermore, several fruit/vegetable samples were successfully employed for evaluating this established sensing system.
Introduction
Organophosphorus (OPs) pesticides are one of the most important pesticides, and are extensively used in the world.1,2 It was reported that more than 100 kinds of OPs compounds have been or are being employed as pesticides since 1940's.3 Most of the widely used OPs belong to highly toxic pesticides, which could be easily released into the environment, resulting in serious ecological pollution and food safety problem.4–6 OPs can inhibit the activity of acetylcholinesterase (AChE) via the formation of phosphorylated cholinesterase, lead to in vivo accumulation of acetylcholine. Excessive acetylcholine can cause serious impairment on nerve function and even death.7 Therefore, it is of importance to establish an efficient and sensitive detection method for OPs.Numerous detection methods for OPs have been developed, based on various chemical or biological mechanisms.8–12 Since 1960's, the emergence of chromatographic techniques13–17 have greatly promoted the development of detecting OPs, thereby various chromatographic techniques including gas phase chromatography (GC), liquid phase chromatography (LC) and thin layer chromatography (TLC) have become important detection methods for OPs. Combining with mass spectrum, these methods can substantially improve their sensitivity and accuracy. However, these established analytical methods usually have several disadvantages, such as complicated operation procedures, time consuming, expensive instruments and requiring highly qualified technicians. Obviously, these methods are not suitable for the continuously, rapidly and on-line monitoring of OPs, especially for the emergency cases. Therefore, developing simple, facile and sensitive methods for detecting OPs is highly demanded. It should be noted that much more convenient detection methods based on electrochemical mechanisms and photoluminescence (PL)/UV-vis absorption spectrum techniques have been developed.18–27
Artificial enzyme mimics have recently attracted extensive attentions,28,29 because (1) they can serve as highly stable and low-cost alternatives to natural enzymes; (2) many kinds of artificial enzyme mimics have been found and created. Therefore, a wide range of applications such as serving as peroxidases or oxidases have also been explored.30–36 Catalysts can repeatedly catalyse some certain reactions for many times to generate a large amount of products, especially photoluminescent or colorimetric compounds. Therefore, these catalytic reactions can be conveniently monitored by PL/UV-vis absorption spectra. A signal amplification could be achieved in these systems, which has been successfully employed in the fields of bio/chemosensing.37–40 Numerous investigations focused on metal/metal oxides nanomaterials capable of acting as the peroxidases and oxidase mimics to catalyse a series of substrates.41–45 However, in general two issues exist, (1) the observed peroxidase-like or oxidase-like activity were in public debated to originate from whether the nature of intact nanomaterials themselves or the surface bound and released metal ions; (2) intrinsic instability of these nanomaterials can susceptibly cause the surface oxidation/aggregation, resulting in the decrease and even loss of their catalytic activity. Recently, we found that Fe3+ ions could serve as the excellent peroxidase mimic towards the 3,3′,5,5′-tetramethyl benzidine (TMB)–H2O2 system.46 Our investigations revealed that Fe3+ ions could exhibit much higher peroxidase-like activity than that of Fe3O4 nanoparticles when Fe concentrations were set to be exactly same. Therefore, if low concentration of catalysts (such as at sub μM level) can be employed, it will be believed to be capable of improving the sensitivity of the established catalytic sensing systems.
Herein, the peroxidase-like activity of Fe3+ ions towards the TMB–H2O2 system, and two enzymatic systems, that is, AChE catalyses the hydrolysis of S-acetylthiocholine (ATCh) to produce thiocholine (TCh), and choline oxidase (CHO) catalyses the oxidation of TCh to generate H2O2, were combined together to establish a multi-catalytic system. The resulting H2O2 could activate Fe3+ ions to catalyse the oxidation of TMB to produce a blue product. When OPs were introduced, the enzymatic activity of AChE can be inhibited by OPs due to the specific combination of OPs and AChE. The inhibition could further suppress the oxidation reaction of TMB catalysed by Fe3+ ions, due to producing less H2O2. Therefore, this change could indirectly indicate the amount of OPs. DDT, as one of organochlorine pesticides, could also inhibit the color reaction, similar to those of the tested OPs. The molecular structures of the tested OPs and DDT were showed in Scheme 1. The multi-catalytic mechanism could promise the high sensitivity of this sensing system towards OPs or organochlorine pesticides.
Experimental
Apparatus
UV-vis absorption spectra were recorded by a Thermo Evolution 300 UV-vis absorption spectrophotometer equipped with a temperature controller. pH measurements were made with a FE20-Five Eosy Plus pH meter.Reagents
Fe(NO3)3·9H2O (98.5%, AR), tri(hydroxymethyl) aminomethane (Tris, BR), HCl (36–38%, AR), NaOAc (99%, AR), HOAc (99.5%, AR), Co(NO3)3·6H2O (99%), Cu(NO3)2·3H2O (99–102%), AgNO3 (99.95%), Ni(NO3)2·6H2O (98.5%) and other organic compounds were obtained from GuoYao (Shanghai, China). 3,3′,5,5′-Tetramethyl benzidine (TMB, 99%), choline oxidase (CHO, from Alcaligenes sp.), acetylcholinesterase (AChE, from Electrophorus electricus), acetylthiocholine chloride (ATCh), dimethoate and 4,4′-DDT (5000 μg mL−1 in methanol) were purchased from Sigma-Aldrich. Paraoxon and dichlorvos (DDVP, 1000 μg mL−1 in methanol) were obtained from Aladdin. Ultrapure water was prepared by employing a Millipore system.Procedures
Results and discussion
The color reaction of TMB–H2O2 system
Our recent investigations revealed that, Fe3+ ions possess extremely high peroxidase-like activity towards the TMB–H2O2 system.46 Considering that other transition metal ions maybe have similar catalytic performance, several traditional transition metal ions including Ag+, Co2+, Ni2+ and Cu2+ ions were thus chosen to evaluate their peroxidase-like activity for catalysing the redox reaction of TMB–H2O2 system. Results indicated that, under similar experimental conditions, that is, 200 mM NaOAc–HOAc buffer solution of pH 4.2, 0.5 mM TMB, 4 mM H2O2 and 37 °C were used, the peroxidase-like activity of Fe3+ ions was much higher than those of other tested transition metal ions, even if their concentration were enhanced up to be 25-fold more than that of Fe3+ ions, color reaction was hardly recorded (Fig. 1a). This further supported that Fe3+ ions have distinctive and excellent peroxidase-like activity towards the TMB–H2O2 system. Therefore, Fe3+ ions were chosen as the peroxidase mimic to establish the multi-catalytic sensing system, taken together the two enzymatic systems of AChE and CHO.Firstly, the experimental conditions of the redox reaction of TMB–H2O2 system catalysed by Fe3+ ions were optimized (third catalytic reaction). pH effect exhibited that the catalytic activity of Fe3+ ions decreased with enhancing pH when 200 mM NaOAc–HOAc buffer solution of various pH was employed to control pH of the system (Fig. 1b). Temperature effect revealed that, the catalytic activity of Fe3+ ions increased with enhancing temperature, when temperature was set as the range from 25 °C to 45 °C, however, further increasing temperature, the catalytic activity decreased (Fig. 1c). Furthermore, reaction time effect demonstrated that, with extending the reaction time window, absorbance at 652 nm wavelength increased (Fig. 1d). Therefore, 200 mM NaOAc–HOAc buffer solution of pH 4.2, temperature of 37 °C and time of 15 min were chosen in the Fe3+–TMB–H2O2 system for further experiments.
Optimizing experimental conditions of AChE and CHO catalytic reactions
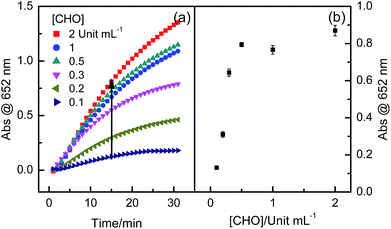
The catalytic hydrolysis reaction of AChE towards ATCh as the substrate was investigated. This was the first catalytic reaction for constructing the multi-catalytic sensing system, due to producing TCh which could act as the substrate for CHO (second catalytic reaction). Considering the enzymatic stability of AChE and CHO, temperature of 37 °C and 50 mM Tris–HCl buffer solution of pH 7.4 were employed in the first and second catalytic reactions. With enhancing AChE concentration from 0.1 to 1 Unit mL−1, absorbance at 652 nm wavelength gradually increased and then showed a platform region at the concentration range from 0.5 to 1.0 Unit mL−1 (Fig. 2). Increasing absorbance was indicative of increasing the amount of color products. The effect of ATCh concentration also exhibited similar observations, that is, when ATCh concentration was enhanced from 30 μM to 500 μM, absorbance at 652 nm wavelength increased. Further enhancing ATCh concentration, absorbance showed somewhat decreasing (Fig. 3). Therefore, 0.5 Units mL−1 AChE and 0.5 mM ATCh were chosen in this multi-catalytic sensing system for other experiments.Considering high selectivity of CHO as an oxidase for the oxidation reaction of TCh, the effect of CHO concentration was also investigated. It was found that, when CHO concentration was enhanced from 0.1 to 0.5 Unit mL−1, absorbance at 652 nm wavelength gradually increased, indicative of producing much more color products; however, with further increasing CHO concentration, the catalytic activity was almost kept as a constant (Fig. 4). It was evident that the producing color intensity of the multi-catalytic sensing system was also dependent on the enzymatic activity of CHO. Therefore, the concentration of CHO was fixed as 0.5 Unit mL−1 for other experiments.
OPs and organochlorine pesticides detection
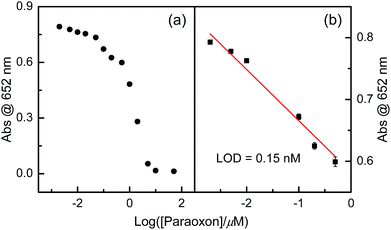
Firstly, paraoxon, DDVP, and dimethoate were chosen as model OPs for exploring the feasibility of the established the multi-catalytic sensing system. In general, the color reaction for detecting OPs could be operated by three steps: (1) various concentration of OPs were incubated with AChE in 50 mM Tris–HCl buffer solution of pH 7.4 for 10 min at 37 °C; (2) the resulting solution of (1) was further mixed with the solutions of CHO and ATCh for 15 min at 37 °C; (3) the resulting solution of (2) was further incubated with Fe3+ ions and TMB solution for 15 min at 37 °C. After the operation procedures of three steps were conducted, the absorbance at 652 nm wavelength was instantly recorded by a UV-vis absorption spectrometer with a certain time interval.OPs concentration-dependent response curves of absorbance at 652 nm wavelength revealed that, with increasing OPs concentration, apparent absorbance gradually decreased (Fig. 5, 7 and 9). Obviously, OPs could suppress this color reaction. It is well known that, AChE can catalyse the hydrolysis of ATCh to generate TCh (the first catalytic reaction), capable of acting as the substrate for CHO (the second catalytic reaction). The second catalytic reaction can produce H2O2. The binding of OPs and AChE can suppress the catalytic activity of AChE towards the hydrolysis of ATCh, which can further result in much less H2O2 than that of the case of in the absence of OPs. Therefore, the amount of introduced OPs can be indirectly indicated by the absorbance at 652 nm wavelength of resulting TMBox.
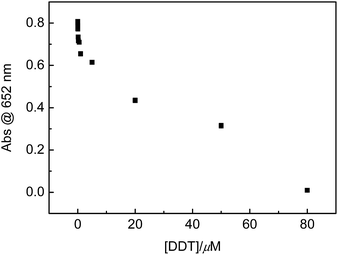
Besides OPs, DDT, as one of banned organochlorine pesticides, is being illegally used. Therefore, DDT was employed for extending the scope of this sensing system. Similar to the OPs cases of paraoxon, DDVP and dimethoate, it was found that the absorbance at 652 nm wavelength of this sensing system was DDT concentration-dependent, too (Fig. 11). That is, with increasing DDT concentration to μM level, absorbance at 652 nm wavelength gradually decreased, indicated that DDT could effectively inhibit the enzymatic activity of AChE. However, on the basis of our knowledge, the inhibition of the catalytic activity of AChE by DDT has not been reported.
Although the relationships between absorbance at 652 nm wavelength and tested OPs or DDT concentrations showed non-linear curves, the linear range could be obtained when OPs or DDT concentrations were transferred into their corresponding logarithms (Fig. 6, 8, 10 and 12) with acceptable regression coefficients. Limit of detections (LODs) of these tested paraoxon, DDVP, dimethoate and DDT were estimated to be 0.15, 0.35, 11 and 0.85 nM, respectively. LODs in this work are lower than those of most of reported cases involving tested OPs or DDT, and the comparison was summarized in Table 1. These results supported that employing multi-catalytic reactions in the sensing system could substantially improve the sensitivity.
| No. | Ref. | Methods | Sensing OPs or DDT | LOD |
|---|---|---|---|---|
| 1 | 44 | Colorimetry | Methyl-paraoxon; dimethoate | 10 nM |
| 5 μM | ||||
| 2 | 27 | Colorimetry | Paraoxon | 200 nM |
| 3 | 47 | Colorimetry | DDVP | 6.7 ppb |
| 4 | 48 | Colorimetry | DDT | 27 ppb |
| 5 | 49 | Fluorometry | Paraoxon | 10 nM |
| 6 | 23 | Fluorometry | DDVP | 4.49 nM |
| 7 | 50 | Luminescence | DDVP | 0.32 μM |
| 8 | 51 | Chromatography | DDT | 0.32–0.51 μg L−1 |
| 9 | 52 | Fluorometry | Paraoxon | 13.1 pM |
| 10 | 22 | Fluorometry | Paraoxon | 8 nM |
| 11 | This work | Colorimetry | Paraoxon | 0.15 nM |
| DDVP | 0.35 nM | |||
| Dimethoate | 11 nM | |||
| DDT | 0.85 nM |
In order to validate the feasibility of this established sensing system for OPs or DDT in practical samples, two group experiments were conducted, (1) judging whether the practical fruit/vegetable samples contain a detectable or no detectable amount of OPs/organochlorine pesticides, (2) the recovery experiments. Results were summarized in Tables 2 and 3.
| Sample | Abs at 652 nm | Results |
|---|---|---|
| a In order to determinate that whether the sample is or not a suspected sample, it was assumed that, when the absorbance at 652 nm wavelength of a sample is lower 10% than that of control experiment, the sample could be judged as a “detectable” sample. If it is not, then it can be called as a “no detectable” sample. Measured Abs@652 nm was obtained by measuring three parallel samples to afford their average value. | ||
| Orange | 0.469 | Detectable |
| Bok-choy | 0.562 | Detectable |
| Pear | 0.618 | Detectable |
| Apple | 0.481 | Detectable |
| Water spinach | 0.616 | Detectable |
| Pleurotus eryngii | 0.768 | No detectable |
| Control experiment | 0.798 | Blank |
| Sample | Spiked/μM | Detected concentration/μM | Recovery/% | RSD |
|---|---|---|---|---|
| a The detected concentration was referred as the average value of the three parallel measurements. After the samples were soaked by 50 mM Tris–HCl buffer solution of pH 7.4, the resulting buffer solution was mixed with a certain volume of DDVP solution of a known concentration, and then the resulting solution was introduced into the sensing system. | ||||
| Pear | 7.2 | 7.39 | 102.6 | 5.4% |
| Bok-choy | 7.2 | 7.22 | 100.3 | 7% |
| Water spinach | 7.2 | 7.12 | 98.9 | 1.7% |
For judging whether the sample is or not a suspected sample, we assume that, when the absorbance at 652 nm wavelength of a sample is lower 10% than that of control blank experiment, the sample could be judged as a “detectable” sample. If it is not, then it can be called as a “no detectable” sample. Of course, allowable absorbance at 652 nm wavelength of the sample can also be lowered some, according to related regulations. Results revealed that five samples could be judged as “detectable” samples and one sample could be judged as “no detectable” sample according to our assumption (Table 2). However, for a practical sample obtained from market, this sensing system could only afford a preliminarily judge whether the sample is a suspected sample, yet could not determinate which kind of OPs or organochlorine pesticides and its content. Accurate detection should be conducted by other methods such as GC and LC. In despite of the drawback, this sensing system is suitable for employing as a powerful tool for preliminarily screening of suspected samples.
DDVP was chosen to further conduct the recovery experiments (Table 3). Results demonstrated that, for three fruit/vegetable samples including pear, Bok-choy and water spinach, the recovery of DDVP was found to locate at the range from 98.9% to 102.6%, when 7.2 μM DDVP solution was added into the soaked solution, with acceptable relative standard deviations (RSDs). This indicated good reliability of this sensing system.
Conclusion
In summary, a simple, facile and sensitive multi-catalytic sensing system has been successfully established based on the peroxidase-like activity of Fe3+ ions, taken together two enzymatic catalytic systems, AChE and CHO. OPs could inactivate AChE, resulting in no production of TCh, which further suppress the formation of H2O2. Therefore, the color reaction of the Fe3+–TMB–H2O2 system could be suppressed, indirectly indicative of the amount of OPs. DDT, one of organochlorine pesticides, showed similar suppression. High sensitivity of this sensing system could be achieved, because LODs of paraoxon, DDVP, dimethoate and DDT were estimated to be 0.15 nM, 0.35 nM, 11 nM and 0.85 nM, respectively. This supports that introducing multi-catalytic reaction could be substantially enhance sensitivity. Several fruit/vegetable samples have been successfully employed to check this established sensing system. Our sensing system showing several merits such as simplicity, rapid operation, and high sensitivity, is expected to be suitable for acting as a powerful tool for preliminarily screening of suspected samples.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 21377124 and 21173268), by the project of new faculty of Huaqiao University (Grant no. 15BS202), and by the State Key Laboratory of NBC Protection for Civilian (Grant no. SKLNBC2013-04 and 2013-05).Notes and references
- M. S. Kim, G. W. Kim and T. J. Park, Biosens. Bioelectron., 2015, 67, 408–412 CrossRef CAS PubMed.
- Q. Liu, X. Jiang, Y. Zhang, L. Zheng, W. Jing, S. Liu and G. Sui, Sens. Actuators, B, 2015, 210, 803–810 CrossRef CAS.
- F. Worek, M. Koller, H. Thiermann and L. Szinicz, Toxicology, 2005, 214, 182–189 CrossRef CAS PubMed.
- Z. Zheng, Y. Zhou, X. Li, S. Liu and Z. Tang, Biosens. Bioelectron., 2011, 26, 3081–3085 CrossRef CAS PubMed.
- Y. Yi, G. Zhu, C. Liu, Y. Huang, Y. Zhang, H. Li, J. Zhao and S. Yao, Anal. Chem., 2013, 85, 11464–11470 CrossRef CAS PubMed.
- X. Yan, H. Li, Y. Yan and X. Su, Food Chem., 2015, 173, 179–184 CrossRef CAS PubMed.
- F. Worek, M. Koller, H. Thiermann and L. Szinicz, Toxicology, 2005, 214, 182–189 CrossRef CAS PubMed.
- Y. He, B. Xu, W. Li and H. Yu, J. Agric. Food Chem., 2015, 63, 2930–2934 CrossRef CAS PubMed.
- A. Chen, D. Du and Y. Lin, Environ. Sci. Technol., 2012, 46, 1828–1833 CrossRef CAS PubMed.
- M. Burnworth, S. J. Rowan and C. Weder, Chem.–Eur. J., 2007, 13, 7828–7836 CrossRef CAS PubMed.
- J. L. Sporty, S. W. Lemire, E. M. Jakubowski, J. A. Renner, R. A. Evans, R. F. Williams, J. G. Schmidt, M. J. V. D. Schans, D. Noort and R. C. Johnson, Anal. Chem., 2010, 82, 6593–6600 CrossRef CAS PubMed.
- G. Aragay, F. Pino and A. Merkoçi, Chem. Rev., 2012, 112, 5317–5338 CrossRef CAS PubMed.
- W. E. Westlake, Anal. Chem., 1963, 35, 105–110 CrossRef.
- L. Mondello, P. Q. Tranchida, P. Dugo and G. Dugo, Mass Spectrom. Rev., 2008, 27, 101–124 CrossRef CAS PubMed.
- A. Fidder, A. G. Hulst, D. Noort, R. de Ruiter, M. van der Schans, H. Benschop and J. P. Langenberg, Chem. Res. Toxicol., 2002, 15, 582–590 CrossRef CAS PubMed.
- F. Hernandez, J. Sancho and O. Pozo, Anal. Bioanal. Chem., 2005, 382, 934–946 CrossRef CAS PubMed.
- S. Yao, A. Meyer and G. Henze, Fresenius' J. Anal. Chem., 1991, 339, 207–211 CrossRef CAS.
- A. P. Periasamy, Y. Umasankar and S.-M. Chen, Sensors, 2009, 9, 4034–4055 CrossRef CAS PubMed.
- G. Liu and Y. Lin, Anal. Chem., 2005, 77, 5894–5901 CrossRef CAS PubMed.
- Y. Wang, J. Jin, C. Yuan, F. Zhang, L. Ma, D. Qin, D. Shan and X. Lu, Analyst, 2015, 140, 560–566 RSC.
- T. Yu, J.-S. Shen, H.-H. Bai, L. Guo, J.-J. Tang, Y.-B. Jiang and J.-W. Xie, Analyst, 2009, 134, 2153–2157 RSC.
- T. Yu, T.-Y. Ying, Y.-Y. Song, Y.-J. Li, F.-H. Wu, X.-Q. Dong and J.-S. Shen, RSC Adv., 2014, 4, 8321–8327 RSC.
- X. Meng, J. Wei, X. Ren, J. Ren and F. Tang, Biosens. Bioelectron., 2013, 47, 402–407 CrossRef CAS PubMed.
- G. Guan, L. Yang, Q. Mei, K. Zhang, Z. Zhang and M.-Y. Han, Anal. Chem., 2012, 84, 9492–9497 CrossRef CAS PubMed.
- K. Zhang, T. Yu, F. Liu, M. Sun, H. Yu, B. Liu, Z. Zhang, H. Jiang and S. Wang, Anal. Chem., 2014, 86, 11727–11733 CrossRef CAS PubMed.
- N. B. Oujji, I. Bakas, G. Istamboulié, I. Ait-Ichou, E. Ait-Addi, R. Rouillon and T. Noguer, Food Control, 2014, 46, 75–80 CrossRef.
- M.-P. N. Bui and A. Abbas, Sens. Actuators, B, 2015, 207, 370–374 CrossRef CAS.
- H. Wei and E. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- Y. Lin, J. Ren and X. Qu, Acc. Chem. Res., 2014, 47, 1097–1105 CrossRef CAS PubMed.
- A. Singh, S. Patra, J.-A. Lee, K. H. Park and H. Yang, Biosens. Bioelectron., 2011, 26, 4798–4803 CrossRef CAS PubMed.
- J. Liu, X. Hu, S. Hou, T. Wen, W. Liu, X. Zhu and X. Wu, Chem. Commun., 2011, 47, 10981–10983 RSC.
- K. S. McKeating, S. Sloan-Dennison, D. Graham and K. Faulds, Analyst, 2013, 138, 6347–6353 RSC.
- W. Shi, Q. Wang, Y. Long, Z. Cheng, S. Chen, H. Zheng and Y. Huang, Chem. Commun., 2011, 47, 6695–6697 RSC.
- G.-L. Wang, X.-F. Xu, L. Qiu, Y.-M. Dong, Z.-J. Li and C. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 6434–6442 CAS.
- Y. Song, K. Qu, C. Zhao, J. Ren and X. Qu, Adv. Mater., 2010, 22, 2206–2210 CrossRef CAS PubMed.
- W. Chen, J. Chen, Y.-B. Feng, L. Hong, Q.-Y. Chen, L.-F. Wu, X.-H. Lin and X.-H. Xia, Analyst, 2012, 137, 1706–1712 RSC.
- N. Graf, M. Göritz and R. Krämer, Angew. Chem., Int. Ed., 2006, 45, 4013–4015 CrossRef CAS PubMed.
- R. Polsky, R. Gill, L. Kaganovsky and I. Willner, Anal. Chem., 2006, 78, 2268–2271 CrossRef CAS PubMed.
- Q. Wu and E. V. Anslyn, J. Am. Chem. Soc., 2004, 126, 14682–14683 CrossRef CAS PubMed.
- C. Panda, B. B. Dhar, B. Malvi, Y. Bhattacharjee and S. S. Gupta, Chem. Commun., 2013, 49, 2216–2218 RSC.
- A. Asati, C. Kaittanis, S. Santra and J. M. Perez, Anal. Chem., 2011, 83, 2547–2553 CrossRef CAS PubMed.
- A. Asati, S. Santra, C. Kaittanis, S. Nath and J. M. Perez, Angew. Chem., Int. Ed., 2009, 48, 2308–2312 CrossRef CAS PubMed.
- H. Jiang, Z. Chen, H. Cao and Y. Huang, Analyst, 2012, 137, 5560–5564 RSC.
- M. Liang, K. Fan, Y. Pan, H. Jiang, F. Wang, D. Yang, D. Lu, J. Feng, J. Zhao, L. Yang and X. Yan, Anal. Chem., 2013, 85, 308–312 CrossRef CAS PubMed.
- W. He, H. Jia, X. Li, Y. Lei, J. Li, H. Zhao, L. Mi, L. Zhang and Z. Zheng, Nanoscale, 2012, 4, 3501–3506 RSC.
- X.-Q. Wu, Y. Xu, Y.-L. Chen, H. Zhao, H.-J. Cui, J.-S. Shen and H.-W. Zhang, RSC Adv., 2014, 4, 64438–64442 RSC.
- R. Pimsen, A. Khumsri, S. Wacharasindhu, G. Tumcharern and M. Sukwattanasinitt, Biosens. Bioelectron., 2014, 62, 8–12 CrossRef CAS PubMed.
- M. Lisa, R. S. Chouhan, A. C. Vinayaka, H. K. Manonmani and M. S. Thakur, Biosens. Bioelectron., 2009, 25, 224–227 CrossRef PubMed.
- X. Ji, J. Zheng, J. Xu, V. K. Rastogi, T.-C. Cheng, J. J. DeFrank and R. M. Leblanc, J. Phys. Chem. B, 2005, 109, 3793–3799 CrossRef PubMed.
- H. A. Azab, A. S. Orabi and A. M. Abbas, J. Lumin., 2015, 167, 360–370 CrossRef CAS.
- Q. Zhou, L. Pang and J. Xiao, J. Chromatogr. A, 2009, 1216, 6680–6684 CrossRef CAS PubMed.
- X. Gao, G. Tang and X. Su, Biosens. Bioelectron., 2012, 36, 75–80 CrossRef CAS PubMed.
Footnote |
| † Y. X. and T. Y. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |