Cross-linked PS-DVB/Fe3O4 microspheres with quaternary ammonium groups and application in removal of nitrate from water†
Zhenliang Fenga,
Wenrui Weia,
Litong Wanga and
Ruoyu Hong*ab
aSchool of Chemical Engineering, Fuzhou University, Fuzhou 350108, China. E-mail: rhong@suda.edu.cn; Fax: +86-512-6588-2057; Tel: +86-512-6588-2057
bCollege of Chemistry, Chemical Engineering and Materials Science & Key Laboratory of Organic Synthesis of Jiangsu Province, Soochow University, SIP, Suzhou 215123, China
First published on 30th October 2015
Abstract
Cross-linked PS-DVB/Fe3O4 microspheres (PFM-3) have been successfully synthesized by a new magnetic colloid swelling polymerization method and further functioned by alternatively using methylamine and 1,4-butanediol diglycidyl as monomers to build a branching polymer with quaternary ammonium groups. The characterization of PFM has been performed by XRD, FT-IR, SEM, TEM and BET surface area analyses. The results showed that PFM-3 possessed a high ion adsorption capacity (149.25 mg g−1) and could adsorb nitrate ions from water efficiently. The material also showed excellent reusability (after washing with sodium chloride, the absorption capacity was little reduced). More importantly, the magnetic microspheres could be removed from waste water easily via introducing an external magnetic field, which made the discoveries in this study provide an effective and environmentally friendly approach for fast removal of nitrate from waste water.
1. Introduction
Due to its high solubility, nitrate contamination is widely dissolved in surface and ground water.1 Nitrate is a threat to the health of humans and animals as it is toxic and could result in many diseases, such as birth defects, spontaneous abortion, increased infant mortality, diarrhea, abdominal pain, vomiting, diabetes, hypertension, respiratory tract infections, changes in the immune system, and methemoglobinemia.2–7 Increased industrial and agricultural activities have resulted in the generation of toxic pollutants, and when they infiltrate into the ground, the concentration of nitrate in ground water would be significantly increased.8–10 Hence, nitrate removal from waste water is essential in the present scenario.How to remove nitrate from water efficiently remains one of the most challenging topics. Studies like ion exchange, biological denitrification, reverse osmosis and electrodialysis have been widely conducted.11–17 Biological denitrification methods using degradation by microorganisms offer the possibility of very specific and selective reduction of nitrate to nitrogen. However, there are some limitations due to contamination of drinking water with germs and metabolic substances, so it is still necessary to extensively recondition the water by filtration and germicidal treatment.16 Ion exchange technology has been identified as a more suitable technology for water decontamination and removal of inorganic ions due to its simplicity, effectiveness, selectivity, recovery and relatively low cost.16–18 Therefore, research on novel adsorbents based on ion exchange technology are attracting global attention considering the practical application. Macroporous styrene-divinylbenzene copolymer is often employed as the host material due to its satisfactory mechanical strength and easy chemical modification.19 For example, Song et al. used macroporous styrene-divinylbenzene as the host material after chloromethylation and following quaternarization to synthetize NDP-2 resin.20 Although those materials have achieved their expected function in selective removal of nitrate from water and demonstrate a more preferable absorption of nitrate ions than towards other anions, such as SO42−, Cl− and HCO3−, there are still some disadvantages, such as difficulty in collection. Since magnetic materials can be easily and efficiently separated from water via introducing an external magnetic field, the application of magnetic materials in water treatment fields, such as heavy metal ion adsorption,21–23 removal of organic contaminants,24–27 and oil spill recovery,28–31 has attracted increasing attention for decades. For example, Mao et al. synthetized magnetic P(St-DVB)/Fe3O4 microspheres with a hollow and porous structure for fast and selective absorption of oils from the surface of water. The oil-absorbed microspheres could be removed from the water surface efficiently by introducing an external magnetic field.32 However, magnetic material with quaternary ammonium groups for the adsorption of anions has seldom been reported before.
In this study, magnetic PS-DVB/Fe3O4 microspheres functioning as a strong basic anion exchange resin were fabricated by a magnetic colloid swelling polymerization process and further functionalized by alternatively using methylamine and 1,4-butanediol diglycidyl as monomers to build a branching polymer with quaternary ammonium groups. The effect of pH on the ion exchange ability and the reusability of PFM were studied. The equilibrium adsorption isotherms of nitrate onto PFM were also investigated. Both Langmuir and Freundlich equations could be used to fit the equilibrium isotherms well.
2. Experimental
2.1. Materials
All chemicals were analytical reagent grade and used without further treatment, and all solutions were prepared with deionized water. Iron(III) chloride hexahydrate (FeCl3·6H2O), iron(II) sulfate heptahydrate (FeSO4·7H2O), aqueous ammonia (25%), azobisisobutyronitrile (AIBN), oleic acid, potassium persulfate (KPS) and sodium dodecyl sulfate (SDS) were received from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Styrene (St), divinylbenzene (DVB) (80%), glycidyl methacrylate (GMA), methylamine (MA, 40% in H2O, v/v) and 1,4-butanediol diglycidyl ether (BDDE, 60% in H2O, v/v) were bought from Aladdin Chemical Co. (Shanghai, China).2.2. Preparation of lipophilic magnetic Fe3O4 nanoparticles
The preparation of lipophilic magnetic Fe3O4 nanoparticles included two steps: firstly, magnetic Fe3O4 nanoparticles were synthesized by means of co-precipitation method.33 FeCl3·6H2O and FeSO4·7H2O were dissolved in deionized water at a molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and put into a flask under nitrogen protection. After stirring for an hour, aqueous ammonia (25%) was quickly added into the solution. The temperature was kept at 80 °C for an hour, and then the pure magnetite particles were prepared. Secondly, oleic acid was added into the above solution. The solution was stirred for another hour under 80 °C, and then the lipophilic magnetic Fe3O4 nanoparticles were prepared.
2 and put into a flask under nitrogen protection. After stirring for an hour, aqueous ammonia (25%) was quickly added into the solution. The temperature was kept at 80 °C for an hour, and then the pure magnetite particles were prepared. Secondly, oleic acid was added into the above solution. The solution was stirred for another hour under 80 °C, and then the lipophilic magnetic Fe3O4 nanoparticles were prepared.
2.3. Preparation of PS-DVB/Fe3O4 microspheres
PS-DVB/Fe3O4 microspheres were prepared by magnetic colloid swelling polymerization method. In the experiment, 200 mL of deionized water and 5 g of self-made hydrophobic magnetic Fe3O4 nanoparticles were added in a 500 mL three-necked round bottom flask under nitrogen protection. Then, 5 mL of styrene and 0.5 mL of DVB were dispersed into the above solution. After stirring (200 rpm) for an hour and when the temperature reached 70 °C, 0.1 g of AIBN was added and then the polymerization process started. The solution was stirred for 10 hours, and then the PS-DVB/Fe3O4 microspheres were prepared. The resulting magnetic polymeric microspheres were magnetically separated after the addition of ethanol and washed 5 times with 50 mL of ethanol, and then dried in a vacuum oven at 60 °C for 24 h. The resulting microspheres were marked as PS-DVB/Fe3O4 particles.2.4. Modified PS-DVB/Fe3O4 microspheres with GMA
5 g of PS-DVB/Fe3O4 microspheres and 1 mL of GMA were dispersed in 200 mL of ethanol. After stirring for 1 hour under nitrogen protection, 10 mL of ethanol solution with 0.1 g of SDS was slowly added into the above solution, then the solution was kept under 70 °C with stirring (200 rpm) for 24 hours. After the reaction had completed, the resulting magnetic polymeric microspheres were magnetically separated after the addition of ethanol, and then washed 5 times with 50 mL of ethanol, and finally dried in a vacuum oven at 60 °C for 24 h.2.5. Synthesis of PFM
GMA modified PS-DVB/Fe3O4 microspheres were quaternary ammonium modified by a multi-step synthesis process. The basic condensation polymerization chemistry depended on MA and BDDE as monomers to build a branching polymer that has quaternary ammonium groups. 500 mg of GMA modified PS-DVB/Fe3O4 was added into 20 mL of MA (4%, v/v) and heated to 65 °C for 30 min; next 20 mL of BDDE (10%, v/v) was added to the above solution and heated to 65 °C for 30 min. In each step, the brown solid was magnetically separated and rinsed with deionized water. The final two steps were repeated 1, 2 and 3 times to make 2, 3 and 4 layers of polymer on PFM, respectively. After being washed three times with 50 mL of 0.6 M NaCl solution and dried in a vacuum oven at 60 °C for 24 h, the PFM with different numbers of bonded layers and positive charge were obtained.2.6. Batch adsorption experiments
Batch adsorption experiments were carried out in 150 mL conical flasks according to the detailed experimental procedure described by Milmile and Chen et al.1,34 The PFM (0.1 g) was placed in contact with nitrate in solution (50 mL) with different concentrations (50, 100, 200, 400 and 600 mg L−1, respectively) for adsorption isotherm study at 293 K separately. The flasks were then transferred to an incubator shaker and vibrated at 140 rpm for 24 h to ensure adsorption equilibrium. The effect of pH on the ion exchange ability and the reusability of PFM was also studied. All batch experiments were repeated at least three times under the same conditions and all the data that appear in this article were the average value.2.7. Characterization
Fourier transformation infrared (FT-IR) spectroscopy was carried out using the potassium bromide (KBr) disk method by a Nicolet Avatar 360 Fourier transform infrared spectrometer to analyze a series of products in the process of synthetizing PFM. The structure of a series of products was investigated by X-ray diffraction (XRD) on a Bruker D8 ADVANCE X-ray diffractometer at a voltage of 40 kV with Cu Kα radiation (λ = 1.5406 Å) in the 2θ range from 10 to 80 degrees. Scanning electron microscopy (S-4700, FE-SEM) with an acceleration voltage of 15 kV and transmission electron microscopy (TEM, Hitachi H-600-II, Japan) was conducted at 200 kV to characterize the morphology of PS-DVB/Fe3O4 and PFM. For TEM sample preparation, the products were dispersed in anhydrous ethanol by ultrasonication for 10 min and then fixed on a carbon-coated copper grid (FCF400-Cu). The magnetic properties were measured at room temperature using a BHV-55 vibrating sample magnetometer. The specific surface areas of TiO2 samples were measured by nitrogen adsorption at 77.5 K (Micromeritics Tristar ASAP 2020) using the Brunauer–Emmett–Teller (BET) method. The nitrate concentration was determined by using Shimadzu model ion chromatography equipment (IC).3. Results and discussion
3.1. Characterization of PFM
To confirm the presence of crystalline magnetite within the polymer particles, the structure of the magnetic polymer particles was characterized by XRD. The XRD patterns of bare Fe3O4 nanoparticles, PS-DVB/Fe3O4 and PFM-3 particles are illustrated in Fig. 1. All the diffraction peaks match the six diffraction peaks well at (220), (311), (400), (422), (511) and (440) by comparing with the Joint Committee on Powder Diffraction Standards (JCPDS card, file no. 79-0418), which were indexed to the cubic spinel phase of Fe3O4. The peak positions of Fe3O4 nanoparticles were unchanged before (black line) and after encapsulation by PS-DVB (red line), as well as functionalized by quaternary ammonium groups (blue line), which illustrates that the crystalline structure of Fe3O4 nanoparticles was not varied during the synthesis process of magnetic polymeric particles. In addition, the broad peak at about 18 degrees might be due to the existence of PS-DVB (according to the red line) and PS-DVB with quaternary ammonium groups (according to the blue line). | ||
| Fig. 1 XRD diffraction pattern of (a) oleic acid coated Fe3O4 nanoparticles, (b) PS-DVB/Fe3O4, (c) PFM-3. | ||
Fig. 2 shows the FT-IR spectra of pure Fe3O4, PS-DVB/Fe3O4 and PFM-3 particles. The adsorption peak at 583 cm−1 is the characteristic absorption of the Fe–O bond, which confirmed the presence of Fe3O4 nanoparticles. The peaks at 3038 cm−1 and 1597 cm−1 correspond to the stretching vibration of aromatic groups, the peak at 2925 cm−1 corresponds to the methylene group, and the three adsorption peaks at 1597, 1446 and 1500 cm−1 are due to the vibration of C–C bonds in the benzene ring,32 which indicated the characteristic peaks of the PS-DVB according to the red line. Compared with the red line, a strong peak at 1114 cm−1 appeared according to the blue line, which corresponds to the stretching vibration of the C–O–C group of polyelectrolytes.35 The FTIR results thus suggested that polyelectrolyte chains were covalently grafted onto the PS-DVB/Fe3O4 microspheres.
The morphologies of the PS-DVB/Fe3O4 microspheres were investigated by TEM (ESI-1(a)†) and SEM (ESI-1(b)†). ESI-1(a)† shows a photograph of magnetic PS-DVB/Fe3O4 composite microspheres prepared by the method of magnetic colloid swelling polymerization after magnetic separation. It can be seen from the TEM images that these microspheres had excellent monodispersibility and all the composite particles were spherical in shape and the magnetite particles were encapsulated by PS-DVB. The average size of the PS-DVB/Fe3O4 composite microspheres was approximately 0.7 μm. ESI-1(b)† shows a photograph of magnetic PS-DVB/Fe3O4 composite microspheres by SEM. Specifically, a much wrinkled and rough topography presented on the surface of PS-DVB/Fe3O4 microspheres. It might be that some small size PS-DVB/Fe3O4 microspheres attached on the surface of the big ones. Although it may not be beautiful, it does increase the specific surface area. ESI-2(a)–(c)† show the morphology of PFM-2 PFM-3 and PFM-4, respectively. The diameter of PFM was about 0.7 μm, almost similar to PS-DVB/Fe3O4 microspheres. An interesting phenomenon was found by comparing these three images; the monodispersibility of PFM decreased with the increasing layer of quaternary ammonium groups, which might be due to the interaction between the quaternary ammonium groups.
The typical room-temperature magnetization curves of the bare Fe3O4 particles (Fig. 3(a)), oleic acid coated Fe3O4 particles (Fig. 3(b)), PS-DVB/Fe3O4 microspheres (Fig. 3(c)) and PFM-3 (Fig. 3(d)) recorded with VSM are illustrated in Fig. 3. As shown in the figure, the magnetization of the samples would approach the saturation values when the applied magnetic field was between ±5000 Oe. Neither remanence nor coercivity was observed, indicating that the magnetic particles produced were super paramagnetic and the single-domain magnetite nanoparticles remained in the polymer microspheres. The saturation magnetization of pure Fe3O4 nanoparticles, oleic acid coated Fe3O4 particles, PS-DVB/Fe3O4 microspheres and PFM-3 was about 66.9, 61.8, 36.4 and 29.1 emu g−1, respectively. The saturation magnetization of the nanoparticles was significantly less than that of bulk magnetite, which was 84 emu g−1. The lower measured saturation magnetization could be due to the smaller size of the iron oxide nanoparticles and the low saturation magnetization of PS-DVB/Fe3O4 composite particles might be attributed to Fe3O4 nanoparticles being wrapped up by polymer,36 which added the thickness of the polymer layer on the surface of the magnetite nanoparticles. The magnetic PS-DVB/Fe3O4 composite particles and PFM-3 can be separated from the aqueous solution under an external magnetic field, and could also be re-dispersed into the aqueous solution with agitation, as shown in the inset of Fig. 3. The magnetic PFM-3 aqueous solution was brown and homogeneous, as shown in the inset (b) of Fig. 3. When the external magnetic field was applied, the PFM-3 was separated from the aqueous solution and the aqueous solution became clear, as shown in the inset (d) of Fig. 3.
In the process of ion exchange, the specific surface area of the adsorbent would dominate a main effect. From BET adsorption isotherms, the specific surface area was calculated according to the method presented in the literature and the data are shown in Table 1.37 It was found that the specific surface area of the PFM decreased with the increasing of the layer of quaternary ammonium groups, and it may be due to the interaction between the quaternary ammonium groups, which were consistent with the result of the SEM analysis.
| Property | BET surface area (m2 g−1) | Micropore area (m2 g−1) | Average pore diameter (nm) | Pore volume (ml g−1) |
|---|---|---|---|---|
| PFM-1 | 34.58 | 3.21 | 9.32 | 0.084 |
| PFM-2 | 29.27 | 2.54 | 8.54 | 0.075 |
| PFM-3 | 23.56 | 2.16 | 6.97 | 0.063 |
| PFM-4 | 15.41 | 1.27 | 5.02 | 0.042 |
3.2. Preparation process of PFM
The formation mechanism of PFM was described as schematically shown in ESI-3.† Initially, magnetic Fe3O4 nanoparticles were synthesized by means of co-precipitation method and then the nanoparticles were modified by oleic acid, which led to the nanoparticles becoming lipophilic. Secondly, the magnetic PS-DVB/Fe3O4 microspheres were prepared by the method of magnetic colloid swelling polymerization. In this process, the oleic acid coated PS-DVB/Fe3O4 could mix with the ST and DVB well with mechanical stirring to form small drops, which were hydrophobic to the surrounding water phase. After the initiator was added into the solution, the polymerization started and the PS-DVB/Fe3O4 was obtained. At last, the PS-DVB/Fe3O4 microspheres were functionalized by quaternary ammonium groups. To start the process, epoxy groups were introduced onto the surface of PS-DVB/Fe3O4 by modifying it with GMA. ESI-4† showed the following multi-step synthesis of PFM. In step (A), MA was reacted with epoxy groups by simple epoxy ring-opening reaction, and the secondary amine groups were obtained on the surface of PS-DVB/Fe3O4. Subsequently in step (B), a layer of cationic polyelectrolytes was formed by the reaction between the secondary amine groups and BDDE. Since the polyelectrolyte chain ends were terminated by glycidyl groups, secondary amine groups could be obtained on the surface of PS-DVB/Fe3O4 by another epoxy ring-opening reaction with MA (C). For the subsequent quaternization reaction, more secondary amine groups were grafted onto the cationic polyelectrolytes. A defined number of bonded layers with desirable positive charge could be grafted onto the surface of PS-DVB/Fe3O4 by repeating steps (B) and (C) alternately.343.3. Equilibrium adsorption studies
Equilibrium adsorption isotherms of nitrate onto PFM were investigated. After the adsorption experiment, the magnetic microsphere could be removed from water easily via introducing an external magnetic field and the separation method became easier than centrifugation. The amounts adsorbed at equilibrium (Qe, mg g−1) were further calculated by eqn (1).38
 | (1) |
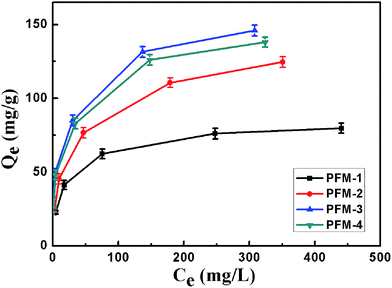 | ||
| Fig. 4 Equilibrium adsorption isotherms of nitrate on PFM-1, PFM-2, PFM-3 and PFM-4 from model solutions at 293 K. | ||
Nitrate adsorption isotherms onto NDP-2 resin were determined in the single-component solution and the experimental data were further correlated by the Langmuir (eqn (2)) and Freundlich adsorption isotherm models (eqn (3)).1
 | (2) |
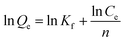 | (3) |
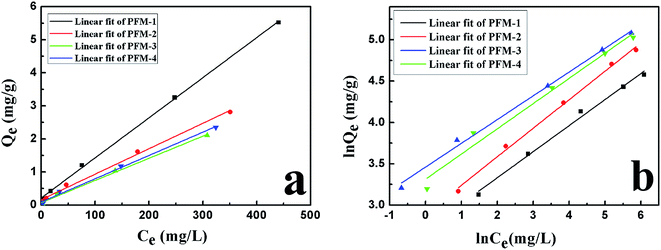 | ||
| Fig. 5 (a) Equilibrium Langmuir isotherm and (b) Freundlich isotherm for nitrate adsorbed using PFM-1, PFM-2, PFM-3 and PFM-4. | ||
| Adsorbent | Langmuir equation | Freundlich equation | ||||
|---|---|---|---|---|---|---|
| Q0 (mg g−1 dry resin) | b (L mg−1) | R2 | n | Kf (mg g−1) | R2 | |
| PFM-1 | 82.51 | 0.056 | 0.999 | 3.17 | 14.90 | 0.992 |
| PFM-2 | 130.04 | 0.046 | 0.994 | 2.90 | 18.13 | 0.996 |
| PFM-3 | 149.25 | 0.090 | 0.993 | 3.48 | 31.85 | 0.995 |
| PFM-4 | 141.84 | 0.073 | 0.995 | 3.28 | 27.51 | 0.974 |
The pH of the aqueous solution was identified as one of the most important variables in the batch adsorption studies. The effect of different pHs was studied and the results are shown in Fig. 6(a). Before the addition of the resin, the initial pH of the solution was 6.24, and then the solution pH was varied from 5 to 10 by adding 0.01 M HCl or 0.01 M NaOH. As depicted in Fig. 6(a), the Qe very slightly decreased for pHs varying from 5 to 10, which indicated that the considerable adsorption of nitrate was independent of the pH for the PFM-3 resin. The final pH of the aqueous solution barely changed after the ion-exchange and removal of resin. The chloride ion on the PFM was exchanged by nitrate ion and the charge balance of the aqueous solution had not been broken. So the final pH of the aqueous solution did not change. Most attractively, at different locations where the pH of the water varied, the PFM-3 resin would exhibit almost the identical adsorption activity independent of the pH. Considering the complex and variable polluted water system, the PFM-3 resin has broad prospects for practical application.
 | ||
| Fig. 6 (a) Effect of varied PH on anion-exchange capacity of PFM-3. (b) Anion-exchange capacity of PFM-3 after different anion-exchange cycles. | ||
The reusability of ion exchange was one of the most important evaluation criteria. After the adsorption experiment, the microspheres could be regenerated by ultrasonic washing with 0.6 M NaCl solution three times and then drying in a vacuum oven. Fig. 6(b) shows the reusability of PFM-3 under different concentrations of nitrate after different adsorbent cycles. We can see that the anion-exchange capacity slightly decreased in the first cycles and barely changed after the first cycle. The decrease in anion-exchange capacity was probably caused by residual nitrate in the pores of the microspheres. The excellent recycling property potentially makes PFM-3 microspheres a cost-effective material.
4. Conclusion
A novel PFM-3 resin was synthesized successfully by a new magnetic colloid swelling polymerization method and then functionalized by quaternary ammonium groups. The PFM-3 showed a higher adsorption capacity of nitrate (149.25 mg g−1) and exhibited almost identical adsorption activity independent of the varied pH. In addition, these microspheres showed excellent recycling property whereby they could keep in a good state even after being used repeatedly. Most importantly, this resin could be removed from solution easily via introducing an external magnetic field. So, it could be widely applied in purification of polluted drinking water resources in the near future.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, No. 21246002), Technology Innovation Foundation of MOST (No. 11C26223204581), Natural Science Foundation of Jiangsu Prov. (No. BK2011328), 333 Talent project (2013) of Jiangsu Prov., and Minjiang Scholarship of Fujian Prov.Notes and references
- S. N. Milmile, J. V. Pande, S. Karmakar, A. Bansiwal, T. Chakrabarti and R. B. Biniwale, Desalination, 2011, 276, 38–44 CrossRef CAS.
- P. K. Singh, S. Banerjee, A. L. Srivastava and Y. C. Sharma, RSC Adv., 2015, 5, 35365–35376 RSC.
- J. B. Heredia, J. R. Domínguez, Y. Cano and I. Jiménez, Appl. Surf. Sci., 2006, 252, 6031–6035 CrossRef.
- A. Azizullah, M. N. K. Khattak, P. Richter and D. P. Häder, Environ. Int., 2011, 37, 479–497 CrossRef CAS PubMed.
- M. H. Ward and J. D. Brender, Environ. Health, 2011, 4, 167–178 Search PubMed.
- J. A. Camargo and Á. Alonso, Environ. Int., 2006, 32, 831–849 CrossRef CAS PubMed.
- J. Colman, G. E. Rice, J. M. Wright, E. S. Hunter, L. K. Teuschler, J. C. Lipscomb, R. C. Hertzberg, J. E. Simmons, M. Fransen, M. Osier and M. G. Narotsky, Toxicol. Appl. Pharmacol., 2011, 254, 100–126 CrossRef CAS PubMed.
- Y. Tong and Z. He, RSC Adv., 2014, 4, 10290–10294 RSC.
- B. Basso and J. T. Ritchie, Agric., Ecosyst. Environ., 2005, 108, 329–341 CrossRef.
- M. Maeda, B. Zhao, Y. Ozaki and T. Yoneyama, Environ. Pollut., 2003, 121, 477–487 CrossRef CAS PubMed.
- N. Mehrabi, M. Soleimani, M. M. Yeganeh and H. Sharififard, RSC Adv., 2015, 5, 51470–51482 RSC.
- S. P. Suriyaraj, M. M. Pillai, A. Bhattacharyya and R. Selvakumar, RSC Adv., 2015, 5, 68420–68429 RSC.
- M. Boumediene and D. Achour, Desalination, 2004, 168, 187–194 CrossRef CAS.
- L. Yuan and T. Kusuda, J. Appl. Polym. Sci., 2005, 96, 2367–2372 CrossRef CAS.
- H. Jiang, P. Chen, S. Luo, X. Tu, Q. Cao and M. Shu, Appl. Surf. Sci., 2013, 284, 942–949 CrossRef CAS.
- C. Jacob, Desalination, 2007, 205, 47–52 CrossRef CAS.
- A. Bhatnagar and M. Sillanpää, Chem. Eng. J., 2011, 168, 493–504 CrossRef CAS.
- S. K. Nataraj, K. M. Hosamani and T. M. Aminabhavi, J. Appl. Polym. Sci., 2006, 99, 1788–1794 CrossRef CAS.
- Z. L. Feng, W. R. Wei, L. T. Wang and R. Y. Hong, Appl. Surf. Sci., 2015, 357, 759–765 CrossRef CAS.
- H. Song, Y. Zhou, A. Li and S. Mueller, Desalination, 2012, 296, 53–60 CrossRef CAS.
- H. Yan, L. Yang, Z. Yang, H. Yang, A. Li and R. Cheng, J. Hazard. Mater., 2012, 229, 371–380 CrossRef PubMed.
- S. Zhang, Y. Zhang, G. Bi, J. Liu, Z. Wang, Q. Xu, H. Xu and X. Li, J. Hazard. Mater., 2014, 27, 27–34 CrossRef PubMed.
- Z. Hua, B. Yang, W. Chen, X. Bai, Q. Xu and H. Gu, Appl. Surf. Sci., 2014, 317, 226–235 CrossRef CAS.
- E. T. Ozer, B. Osman, A. Kara, N. Besirli, S. Gucer and H. Sozeri, J. Hazard. Mater., 2012, 229, 20–28 CrossRef PubMed.
- H. Y. Zhu, R. Jiang, L. Xiao and W. Li, J. Hazard. Mater., 2010, 179, 251–257 CrossRef CAS PubMed.
- Q. Liu, L. Wang, A. Xiao, J. Gao, W. Ding, H. Yu, J. Huo and M. Ericson, J. Hazard. Mater., 2010, 181, 586–592 CrossRef CAS PubMed.
- Y. Tai, L. Wang, J. Gao, W. A. Amer, W. Ding and H. Yu, J. Colloid Interface Sci., 2011, 360, 731–738 CrossRef CAS PubMed.
- A. Pavıa-Sanders, S. Zhang, J. A. Flores and K. L. Wooley, ACS Nano, 2013, 7, 7552–7561 CrossRef PubMed.
- Q. Zhu, F. Tao and Q. Pan, ACS Appl. Mater. Interfaces, 2010, 2, 3141–3146 CAS.
- L. Zhu, C. Li, J. Wang, H. Zhang, J. Zhang, Y. Shen, C. Li, C. Wang and A. Xie, Appl. Surf. Sci., 2012, 258, 6326–6330 CrossRef CAS.
- M. Chen, W. Jiang, F. Wang, P. Shen, P. Ma, J. Gu, J. Mao and F. Li, Appl. Surf. Sci., 2013, 286, 249–256 CrossRef CAS.
- J. Mao, W. Jiang, J. Gu, S. Zhou, Y. Lu and T. Xie, Appl. Surf. Sci., 2014, 317, 787–793 CrossRef CAS.
- X. Liu, H. Liu, J. Xing, Y. Guan, Z. Ma, G. Shan and C. Yang, China Particuol., 2003, 1, 76–79 CrossRef CAS.
- Y. L. Chen, B. C. Pan, H. Y. Li, W. M. Zhang, L. Lv and J. Wu, Environ. Sci. Technol., 2010, 44, 3508–3513 CrossRef CAS PubMed.
- Z. Huang, L. Xi, Q. Subhani, W. Yan, W. Guo and Y. Zhu, Carbon, 2013, 62, 127–134 CrossRef CAS.
- M. Yamaura, R. L. Camilo, L. C. Sampaio, M. A. Macêdo, M. Nakamura and H. E. Toma, J. Magn. Magn. Mater., 2004, 279, 210–217 CrossRef CAS.
- Y. Chen, Z. Qian and Z. C. Zhang, Colloids Surf., A, 2008, 312, 209–213 CrossRef CAS.
- K. S. W. Sing, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- J. N. Wang, Y. Zhou, A. M. Li and L. Xu, J. Hazard. Mater., 2010, 176, 1018–1026 CrossRef CAS PubMed.
- B. H. Gu, Y. K. Ku and P. M. Jardine, Environ. Sci. Technol., 2004, 38, 3184–3188 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19642f |
| This journal is © The Royal Society of Chemistry 2015 |


