Controllable growth of NiCo2O4 nanoarrays on carbon fiber cloth and its anodic performance for lithium-ion batteries†
Abstract
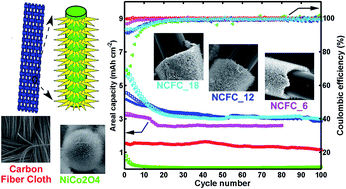
Flexible materials are promising materials for wearable devices and have attracted much attention. Among these, carbon fiber cloth (CFC) is a soft carbon substrate on which it is suitable to grow metal oxide nanoarrays. Meanwhile, a high areal mass loading of an electrode is of great significance in practice. Consequently, this work fabricated high areal mass loading NiCo2O4 nanoarrays by direct growth onto a CFC without binder and conductive additives. In the experiments, the thickness of the NiCo2O4 layer could be controlled to 1.8–4.4 μm by varying the hydrothermal reaction time between 6–18 hours. Since a high areal mass loading of an electrode is of great significance in practice, the thickness of NiCo2O4 was controlled to 4.4 μm using an 18 hour hydrothermal reaction time. The electrochemical behavior of the NiCo2O4/CFC anode was evaluated with 10 cycles at five different current rates from 100 to 500 mA g−1 in sequence and another 100 cycles at 100 mA g−1. It had a reversible areal capacity of 2.39 mA h cm−2 (799 mA h g−1) at the 150th cycle when the current density was 0.299 mA cm−2 (100 mA g−1). The high areal capacity was ascribed to the larger NiCo2O4 mass loading on the CFC substrate. The one-dimensional flexible conductive carbon fiber backbone and the free-standing NiCo2O4 nanoarrays on it also released the stress generated from the discharge/charge process. The nanostructure of NiCo2O4/CFC in this work offers great benefits for high areal capacity anode materials.

 Please wait while we load your content...
Please wait while we load your content...