Pressure-induced structural changes and elemental dissociation of cadmium and mercury chalcogenides†
Abstract
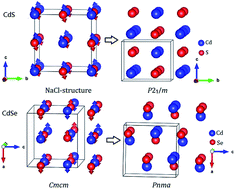
Cadmium and mercury chalcogenide compounds have broad applications (e.g. in light-emitting diodes, chemical sensors, and solar cells). Pressure has profound effects on their structures and properties. However, their structures and phase transition sequences under pressure have not been fully determined, which greatly hinders their further applications. Here, we have extensively explored the high-pressure structures of cadmium and mercury chalcogenides (CdX, HgX, X = S, Se and Te) by using the in-house developed CALYPSO method. The results show that their high-pressure structures and phase transition sequences strongly correlate with the chalcogens. An intriguing dissociation into Cd (or Hg) + X under strong compression was observed. This observation might originate from the special sandwich structure, which significantly frustrates Cd (or Hg)–X bonding and enhances the interaction of the same kind of atoms. Moreover, the dissociation pressures of cadmium and mercury chalcogenides decrease with increasing the atomic radius of the chalcogens. The high-pressure decomposition of cadmium and mercury chalcogenides might have important implications for other binary compounds.

 Please wait while we load your content...
Please wait while we load your content...