The effect of silica sols on electrodeposited zinc coatings for sintered NdFeB†
Abstract
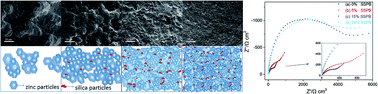
We developed an in situ method to prepare silica particle-containing zinc coatings on NdFeB. Silica sols with silica nanoparticles of 30–150 nm in diameter were prepared through the hydrolysis and condensation of tetraethyl orthosilicate with an acid catalyst. Zinc coatings were prepared on NdFeB magnets using plating baths that contained the silica sols. The effect of the volume content of silica sols on the zinc coatings was studied via scanning electron microscopy (SEM), X-ray diffraction (XRD) and electrochemical analysis. The SEM results showed that the silica nanoparticles were embedded in the zinc particles. Compared with the zinc coatings that were electroplated in the other plating baths, the surface morphology of the zinc coating formed when the silica sol content in the plating bath was in a proper range exhibited better uniformity. The decrease in the corrosion current density value and the increase in the impedance value that were measured via electrochemical testing demonstrated that the embedded silica nanoparticles, which served as corrosion inhibitors, improved the corrosion resistance capability of the zinc coatings on the NdFeB magnets.

 Please wait while we load your content...
Please wait while we load your content...