Multicolour electroluminescence from light emitting diode based on ZnO:Cu/p-GaN heterojunction at positive and reverse bias voltage
Abstract
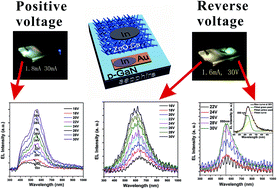
Incorporation of Cu atoms in ZnO can arouse Cu-related defect, which will influence electroluminescence (EL) light of light emitting diode (LED) based on ZnO:Cu/p-GaN heterojunction. ZnO:Cu nanobushes were grown on p-GaN film by simple chemical vapour deposition (CVD) method. Ultraviolet (UV) photoluminescence (PL) peak of ZnO:Cu had a redshift compared with that of pure ZnO, and low temperature (5 K) PL spectrum exhibited a structured green emission, indicating the substitutional incorporation of Cu atoms in ZnO crystal lattice. The LED based on ZnO:Cu/p-GaN heterojunction emitted blue-greenish EL light at p-GaN site at the positive bias voltage, and green EL peak gradually overtook the blue EL peak with the increase of positive bias voltage. At reverse bias voltage, this LED not only exhibited yellow-green EL light at ZnO:Cu site, but also emitted from orange to yellow EL light at p-GaN site with the increase of reverse bias voltage. The origins of EL light at positive and reverse bias voltage were explained using PL spectra and energy band diagram of ZnO:Cu/p-GaN structure.

 Please wait while we load your content...
Please wait while we load your content...