Photoinduced curcumin derivative-coatings with antibacterial properties†
M. Condata,
P.-E. Mazeranb,
J.-P. Malvalc,
J. Lalevéec,
F. Morlet-Savaryc,
E. Renarda,
V. Langloisa,
S. Abbad Andalloussid and
D.-L. Versace*a
aInstitut de Chimie et des Matériaux Paris-Est, Equipe Systèmes Polymères Complexes, UMR 7182, CNRS-Université Paris-Est Créteil Val de Marne 2-8 rue Henri Dunant, 94320 Thiais, France. E-mail: versace@icmpe.cnrs.fr; Fax: +33 1 49 78 12 01; Tel: +33 1 49 78 12 28
bLaboratoire Roberval, UMR CRNS-UTC 7337, Centre de Recherche de Royallieu, Université de Technologie de Compiègne, 60205 Compiègne Cedex, France
cInstitut de Science des Matériaux de Mulhouse, IS2M-LRC 7228, 15 rue Starcky, 68057 Mulhouse, France
dUnité Bioemco Equipe IBIOS, UMR 7618 CNRS - Université Paris-Est Créteil Val-de-Marne, 61, Avenue Général de Gaulle, 94010 Créteil Cedex, France
First published on 29th September 2015
Abstract
The development of new antibacterial coating (against Escherichia coli and Staphylococcus aureus) with the use of a natural dye (curcumin) and epoxidized soybean oil, according to a photochemistry process has been investigated. Curcumin has been used both as a photosensitizer and an antibacterial agent under visible light illumination. The photoinduced coatings show good adherence properties on an inox substrate and a high thermal stability to 375 °C. Under visible light activation, singlet oxygen (1O2) could be generated from the curcumin derivative-coatings, thus inhibiting by 99% and 95% the growth of Staphylococcus aureus and Escherichia coli, respectively, even after 48 h of incubation.
1. Introduction
Infections by pathogenic microorganisms are of great concern in many fields, particularly in medical devices, hospital surfaces/furniture, and surgery equipment. Approximately 64% of hospital-acquired infections worldwide are due to the attachment of bacteria to medical implants, and they are associated with an annual mortality of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 persons in the US as well as an increase in health-care costs.1,2
000 persons in the US as well as an increase in health-care costs.1,2
Persistence of clinically relevant bacteria on inanimate surfaces can remain up to several days and even months.3 It is of great importance to interrupt this transmission pathway by a self-disinfecting effect of surfaces. To solve the problem of increasing resistance of bacteria much attention has been focused on developing new antimicrobial systems in biomedical industry. Therefore, new chemical strategies have been developed to prevent the bacterial colonization of materials surface. Four approaches could be cited: the first approach focused on reducing the capacity of bacteria to attach onto a surface.4–8 The second approach is based on lethal contact which induces the biochemical death of bacteria.9–11 The third approach has referred to the biocide leaching.12–16 A final alternative method for disinfecting surfaces consists in using a coating that produces reactive oxygen species upon light activation, also called the method of photodynamic inactivation (PDI). In this method, a photosensitizer (PS) is excited by light, for further generating reactive oxygen species17 (e.g. singlet oxygen, 1O2), that can cause cell death.18–21 In this context, PSs do not have to penetrate the bacterium or even come into a contact with the cell in order to be effective.
Antibacterial surfaces which work with the PDI principle are of great interest due to their preventive character for infections.22–24 Such surfaces could help to reduce the transmission of multi-resistant microorganisms, which is of great importance in hospital hygiene.25 It has been demonstrated that PSs destroy bacteria, viruses, fungi, yeasts and parasites upon illumination.26–28 Gram positive bacteria seem to be however more susceptible to PDI than Gram negative bacteria.29,30 As such surfaces do not necessarily lead to PS consumption, self-disinfecting coatings could offer a constant prevention of microorganism adhesion and proliferation on any surface.31 Many investigations have already been performed with porphyrins and phthalocyanine derivatives,32,33 hydroxyethyl Michler's ketone,34 toluidine blue,35 eosin Y,36,37 methylene blue35,38 or rose bengal.39
To the best of our knowledge, no report has hitherto been published on the synthesis of antibacterial coatings under visible light activation using a natural dye, curcumin, which is used both as a photosensitizer and an antibacterial agent according to an environmentally substainable “photo chemistry” method.
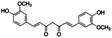
Curcumin, the yellow-orange dye derived from the rhizomes of Curcuma longa, primary bioactive component of turmeric, has recently received attention from chemists due its wide range of potential applications ranging from pharmacology40–43 (e.g. antioxidant, anti-inflammatory, anti-viral, anti-bacterial, anti-fungal, and antitumor activities) to its photobiological and photosensitizing actions.44–46 More interestingly, it has also been reported that curcumin can act as a photoinitiator for free radical47 and cationic48,49 photopolymerizations under air. However, the combination of the anti-bacterial and photosensitizing properties of curcumin has not been used yet for the synthesis of natural antibacterial coatings with highly thermal and mechanical strength.
In this paper, we present a new and simple route to efficiently synthesize an antibacterial coating derived from epoxidized soybean oil which is deposited on stainless steel plate and efficient enough to inhibit the bacteria growth, according to an environmentally-friendly method. The first part of this study demonstrates the photochemical properties of the photoinitiating system (curcumin and iodonium salt) through steady state, phosphorescence and fluorescence experiments. In a second part, a particular effort was made to characterize the synthesized coatings. For this purpose, the thermal and mechanical properties of the coatings were characterized by Thermo Gravimetric Analysis (TGA), nanoindentation and scratch tests. In addition, the generation of fluorescence through the curcumin derived coatings under visible light irradiation was investigated by fluorescence spectrophotometry. To validate the study, the antibacterial property of the synthesized coatings against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) was evaluated.
2. Results and discussion
2.1 Photochemical properties
The absorption spectrum of curcumin in acetonitrile exhibits an absorption maximum at 420 nm (εmax = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) which can be assigned to the π–π* transitions. In order to demonstrate the photosensitizer effect of curcumin onto iodonium salt (Iod), steady state photolysis experiments have been performed. Fig. 1A describes the evolution of UV-visible absorbance of curcumin under different times of irradiation in the visible range. After 120 s of irradiation, a decrease of only 10% of the total absorbance of curcumin was observed at 420 nm.
000 M−1 cm−1) which can be assigned to the π–π* transitions. In order to demonstrate the photosensitizer effect of curcumin onto iodonium salt (Iod), steady state photolysis experiments have been performed. Fig. 1A describes the evolution of UV-visible absorbance of curcumin under different times of irradiation in the visible range. After 120 s of irradiation, a decrease of only 10% of the total absorbance of curcumin was observed at 420 nm.
However when curcumin is irradiated in the presence of Iod (Fig. 1B), the maximum absorbance peak for curcumin at 420 nm strongly decreases. Dramatic photobleaching was observed after only 30 s of irradiation, thus demonstrating that the photoinitiating system curcumin/Iod is highly reactive.
The ability to generate protons using curcumin as visible photosensitizer of Iod is illustrated in Fig. 2. In this experiment, both compounds are mixed together in acetonitrile (ACN) in presence of rhodamine B (RhB) as acid indicator.50 As shown in Fig. 2A, the irradiation of the solution at 420 nm leads to a gradual decrease of the longest wavelength absorption band of curcumin in concomitance with the growth of the lowest energy band of the acid form of RhB at 550 nm. It should be emphasized that the irradiation of Iod without curcumin and in the same conditions does not generate any protons. Reciprocally, the irradiation of curcumin without Iod does not induce any acid generation as depicted in Fig. 2B (see the inset). It should be noted that the presence of Iod (charged molecule) increases the ionic strength force of the solution, thereby leading preferentially to the formation of the rhodamine B in its acid form at t = 0 s (see Fig. 2A). On the contrary, without Iod in solution, RhB remains in its basic form at t = 0 s.
Finally, the presence of oxygen in the solution clearly has a detrimental effect in the efficiency for acid photogeneration. Fig. 3 shows the time evolution of the absorbance at λmax of the acid form of RhB for nitrogen saturated and non degassed solutions. One clearly observes a strong enhancement by a factor 7 for the initial of H+ photogenerated for nitrogen saturated solution. Such an oxygen inhibition effect indirectly suggests that the photosensitization of Iod by excited curcumin mainly proceed through its triplet excited state. The quantum yield for the H+ photogeneration (ΦH+) has been evaluated at 0.01 according to the method reported by Scaiano et al.51
The free energy change ΔGT for the 3curcumin/Iod electron transfer reaction is highly reactive and make the process favourable according to the Rehm–Weller equation52 (eqn (3)): −1.20 V (Eox of curcumin = 0.78 eV as measured by cyclic voltammetry in this work; Ered of Iod = −0.2 V,53 triplet state energy ET of curcumin = 1.95 eV as extracted from the phosphorescence spectrum (Fig. 4).
Fig. 4 displays the time-gated phosphorescence spectrum of curcumin in glassy matrix of ethanol (77 K). The maximum luminescence is located at 636 nm leading to a lowest triplet state energy of ca. 1.95 eV. The phosphorescence lifetime of about 14 μs (see inset Fig. 4) is also a clear indication of an emissive triplet state having strong n,π* character.
Even if the triplet energy transfer between curcumin and Iod seems to be the main process, the singlet energy electron transfer could be a second way according to the fluorescence experiments. The fluorescence emission of curcumin in acetonitrile is described in Fig. 5. The blue curve corresponds to the absorbance spectrum of curcumin in its fundamental state with a maximum absorption at 420 nm. The red curve corresponds to the emission spectrum of curcumin with a maximum at 520 nm. The crossing of the two curves corresponds to a λfluo at 460 nm.
In the presence of Iod, the fluorescence of curcumin at 520 nm is quenched by electron transfer reaction (Fig. 6). A quenching constant kqτ0, between curcumin and Iod, was evaluated at 16.2 ± 0.2 M−1. As the fluorescence lifetime of curcumin45 τ0 was evaluated at 376–695 ps in acetonitrile; therefore kq is around 3 × 1010 M−1 s−1. The large interaction rate constant kq indicates that the process is almost diffusion-controlled.
 | ||
| Fig. 6 Fluorescence quenching of curcumin by Iod in acetonitrile. Inset: evolution of the fluorescence intensity as a function of [Iod]. | ||
The free energy change ΔGS for the 1curcumin/Iod electron transfer reaction is favourable according to the Rehm–Weller equation52 (eqn (1)): −1.72 V (Eox of curcumin = 0.78 eV as measured by cyclic voltammetry in this work; Ered of Iod = −0.2 V,53 singlet state energy ES of curcumin = 2.7 eV as extracted from the UV-vis absorption and fluorescence emission spectra).
2.2 Synthesis of the coatings
For the first time, the photochemical reactivity of the photosensitive formulation containing epoxidized soybean oil/curcumin/Iod was evaluated with the use of RT-FTIR by following the decrease of the epoxy group through visible illumination at 810 cm−1 (Fig. 7A). During irradiation, the intensity of the epoxy group strongly decreases and a new peak at 1080 cm−1, corresponding to the formation of a polyether network, was observed, thus demonstrating the photopolymerization of the epoxidized soybean oil (Fig. 7B). The final epoxy conversion at 600 s is 80%. The coating is totally take-free: an optical image of the coating which is deposited on an inox substrate is displayed on Fig. 8. | ||
| Fig. 8 Optical image of the inox plate (left) and the curcumin derivative-coating deposited on an inox plate. | ||
2.3 Thermo gravimetric analysis (TGA)
Thermal stability of the coatings was studied by Thermal Gravimetric Analysis (TGA), under oxygen atmosphere. The results are displayed in Fig. 9. It can be observed that the coating reached 50% weight loss at 370 °C.2.4 Nanoindentation and scratch tests
The loading and unloading curves during the nanoindentation tests are closed to be superimposed showing that the coatings have at room temperature a quasi-viscoelastic behavior indicating that the polymer is in a rubber-like state at room temperature. The elastic modulus as measured by the Continuous Stiffness Measurement (CSM) method was found to be around 105 ± 12 MPa and 130 ± 12 MPa for the inox substrate and glass substrate respectively (means ± standard deviation). Concerning the scratch tests, the comparison of the height profiles before and after scratch tests reveals the recovery of the material confirming the rubber-like behavior (Fig. 10). A bulge on the height profile during and after scratch evidences the apparition of the brittle fracture of the film. The critical load is 70 ± 27 mN and 58 ± 32 mN for the steel and glass substrate respectively (means ± standard deviation). On glass, brittle fractures of the film do not lead to delamination showing a good adherence to the substrate (Fig. 11A), on the opposite, delamination occurs during scratch test showing a weaker adherence of the film on the substrate in comparison with glass substrate (Fig. 11B).2.5 Fluorescence of the coating
After irradiation of the curcumin derived formulation (600 s), free curcumin dyes left embedded inside the coating as demonstrated by UV-vis spectrometry (Fig. 12) and epifluorescence (Fig. 13). The fluorescence of the film which stems from the remaining curcumin suggests that excitation of this chromophore can be used to efficiently generate singlet oxygen as it will be highlighted in the following section. | ||
| Fig. 12 Evolution of the UV-vis spectra of the curcumin derivative-coating after 600 s of irradiation. Xe lamp, intensity = 30 mW cm−2. Formulation was sandwiched under 2 glass substrates. | ||
2.6 Antibacterial property
Prior to investigate the antibacterial effect of the curcumin derivative-coatings, the ability of curcumin to inhibit the bacterial proliferation in solution was investigated against Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria after 2 h, 6 h, 24 h and 48 h, with and without irradiation in the visible range. Initially, 3 × 106 CFU mL−1 of each bacteria was introduced in solution or put into contact with coatings at t = 0 h.First, a reference study referring to the light irradiation against the two bacteria strains was performed (Fig. 14). The results show that the irradiation does not have any influence on the growth of E. coli and S. aureus. A second reference study was done in the presence of DMSO, because curcumin is not soluble in water. According to the first results, DMSO does not affect the bacterial activity as the decrease of CFU is not significant whatever the stains used.
 | ||
| Fig. 14 Influence of the incubation time (0, 2, 6, 24 and 48 h) and the irradiation on the growth of (a) E. coli and (b) S. aureus. [Curcumin] = 160 μM. | ||
The antibacterial effect of curcumin was then done in DMSO, under light activation. The irradiation of curcumin leads to an antibacterial effect on both strains: indeed, the irradiated curcumin solutions allow the total death of S. aureus after 6 h of incubation and 99% inhibition of E. coli growth after 2 h of incubation. The antibacterial effect against the both bacteria strains remains efficient even after 48 h. The variation in curcumin killing efficacy at different exposure durations was found to be statistically significant (p value < 0.05 on comparing percentage survival). Without irradiation, more than half of bacteria are always alive after 48 h of incubation for both bacteria.
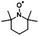
These results confirm the inactivation or lysis of S. aureus and E. coli according to two processes. E. coli, a Gram negative bacteria, have one or a few layers of peptidoglycan and an external membrane with lipoproteic character;35 thus allowing the hydrophobic components (like curcumin) to penetrate the membrane and leading to its alteration by disruption. On the contrary, Gram positive bacteria (S. aureus), protect their cytoplasmic membrane with a thick multilayer peptidoglycan wall that blocks the introduction of hydrophobic components because of the presence of amino acids and sugars within the cell membrane.35 Therefore, only hydrophilic components penetrate the wall. So, a second interesting explanation could be pointed out from our antibacterial results. Some reports concerning the inactivation of bacteria demonstrate that photosensitizers do not have to penetrate the cell membrane or even to come into contact to be effective. Indeed, if sufficient quantities of singlet oxygen can be generated near the outer membrane of the bacteria, it will lead to its damage.54 In our investigation, Electron Paramagnetic Resonance (EPR) spectroscopy was used to monitor the 1O2 generation ability of curcumin upon photo-illumination in the visible range. Amount of 2,2,6,6-tetramethylpiperidine (TEMP), a diamagnetic molecule, was used to capture singlet oxygen by yielding a paramagnetic product, the nitroxide radical TEMPO.17,55,56 As shown in Fig. 15, the EPR spectral signal of three lines of equal intensity, which is attributed to the TEMPO nitroxide radical, was observed when an oxygen-saturated solution of curcumin was irradiated in the presence of TEMP at room temperature. Upon photoirradiation of curcumin, the intensity of the EPR signal increases gradually, indicating the formation of 1O2 molecules.
The quantum yield for singlet oxygen generation (ΦΔ) upon excitation of curcumin has been evaluated in ethanol based on a photoxidation method using 1,3-diphenylisobenzofuran (DBF) as 1O2-sensitive indicator.57 Rose Bengal58 (RB) was employed as a standard photosensitizer (Φ*Δ = 0.68 in ethanol) and isoabsorbant solutions of curcumin and RB solutions were irradiated at 480 nm. Both ethanolic solutions contained DBF at 5 × 10−4 M. The absorbance of DBF at 327 nm was recorded at different irradiation times as displayed in Fig. 16. At 480 nm, DBF weakly absorbs and the absorbance of DBF alone or curcumin alone remains constant under visible light activation as demonstrated in Fig. S1.† The value of ΦΔ/Φ*Δ is obtained from the ratio of the corresponding slopes at initial time of irradiation. A value of 0.05 is therefore measured for the 1O2 photogeneration quantum yield by curcumin.
According to these results, it is likely that the curcumin which have been introduced into the synthesized coatings could generate singlet oxygen to inhibit the bacteria growth on stainless steel substrates. This is well supported by the fact that no curcumin is released from the polymeric coatings after the different incubation times, when coatings are introduced in aqueous bacteria solution. This phenomenon could be explained by the high final polymerization conversion of the epoxidized soybean oil after visible light irradiation.
The ability of the curcumin derivative-coatings (deposited on stainless steel substrates) to inhibit the bacteria adhesion was investigated in comparison with the unmodified stainless steel substrates, and the curcumin-derivative coatings with and without light activation (Fig. 17). A quantitative method for quantifying the total biofilm population has been used as reported by many antibacterial investigations.11,59,60 According to Fig. 17, the antibacterial effect is more efficient against S. aureus than for E. coli under light activation. After 2 h, the number of CFU S. aureus decreases, whereas it strongly increases on the unmodified stainless steel plate. After 24 h and 48 h of incubation, 99% inhibition of S. aureus growth is observed. For E. coli, the antibacterial activity of the coating begins after 6 h of incubation (Fig. 17) but remains less efficient than for S. aureus. Indeed, only 80% and 95% inhibition of E. coli growth are demonstrated after 24 h and 48 h respectively. Another interesting result is the increase of the bacteria population on the curcumin-derivative coatings without visible light illumination and whatever the bacteria strains used. This later result demonstrates that light is essential for leading to an antibacterial effect.
This experiment finally demonstrates the efficiency of the coating to strongly diminish the adherence/proliferation of two bacteria strains on the stainless steel supports by generating reactive oxygen species (1O2 molecule). It is worth noticing that the live/dead assay for evaluating the death of bacteria in this case is not really suitable because of the intense fluorescence of the polymeric coatings due to the presence of curcumin.
3. Experimental
3.1 Materials
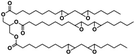
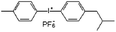
Curcumin (≥65%), epoxidized soybean oil (ESBO), 2,2,6,6-tetramethyl-1-piperidinyloxy (98%, TEMPO), 2,2,6,6-tetramethylpiperidine (≥99%, TEMP), Rose Bengal (RB, 95%), rhodamine B (RhB, ≥ 95%) and 1,3-diphenylisobenzofuran (DBF, 97%) were purchased from Sigma-Aldrich. Iodonium 4-(2-methylpropyl)phenyl-hexafluorophosphate (Iod, Irgacure®250) was purchased from Badische Anilin und Soda Fabrik (BASF). Acetonitrile (ACN), ethanol (EtOH) and toluene were supplied by Sigma-Aldrich (HPLC grade). The main compounds used in this study are displayed in Table 1. Stainless steel substrates were cut on large stainless steel plate to obtain the plate size of 1.5 × 1.5 cm.3.2 Steady state photolysis experiments
Curcumin, in the presence of additive (i.e. Iod) in acetonitrile, was irradiated with the xenon lamp, and the UV-vis spectra were recorded on a Varian spectrophotometer (cary50 bio) in the range 250 nm to 800 nm in acetonitrile.3.3 Determination of the quantum yield acid generation
The method has been previously described by Scaiano et al.51 More precisely, the quantum yields for acid generation were measured under irradiation at 420 nm using a 200 W xenon lamp (Hamamatsu, L8-03) equipped with a band pass. The irradiated curcumin/Iod solution in acetonitrile was previously N2 degassed. The progress of the photoreaction was monitored via UV-vis absorption spectra.The acid generation in acetonitrile was evaluated from a calibration curve of rhodamine B (used as a sensor of photoacid generation) which was gradually protonated by addition of p-toluenesulfonic acid.61 Then, photoacid quantum yields were calculated according to the eqn (1):
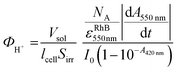 | (1) |
3.4 Electron paramagnetic resonance (EPR) experiments
EPR experiments were carried out using the Bruker EMX plus X-Band EPR Spectrometer. The radicals were generated at room temperature upon LED exposure (420 nm) under air.3.5 Photopolymerization procedure
For the cationic photopolymerization, 6.6 mg of Iod (2.2 wt% with respect to the epoxy monomer) 18.0 mg of curcumin (6 wt% with respect to the epoxy monomer) was dissolved into epoxy monomer formulation (ESBO, 300 mg) containing 150 μL of acetonitrile. Kinetics of photopolymerization were followed by real time Fourier transform infrared spectroscopy (RT-FTIR) using a Thermo-Nicolet 6700 instrument. The liquid samples were applied to a BaF2 chips by means of calibrated wire-wound applicator. The thickness of the UV-curable film was evaluated at 100 μm. The RT-FTIR analyses were carried out under air conditions. Samples were irradiated at room temperature, by means of a Lightningcure LC8-03 lamp from Hamamatsu, equipped with a xenon lamp (200 W) coupled with a flexible light guide. The end of the guide was placed at a distance of 6 cm. The maximum UV light intensity at the sample position was evaluated to be 30 mW cm−2. The photopolymerization was monitored by the disappearance of the epoxy function of the ESBO monomer at 910 cm−1. The decreases of epoxy function at 910 cm−1 and the increasing of polyether at 1080 cm−1 demonstrate the efficiency of the photopolymerization. Conversion rate was calculated with the eqn (2):| Epoxy conversion (%) = (A0 − At)/A0 | (2) |
3.6 Coatings preparation
Prior to the deposition of formulation on stainless steel plate, the later has been intensively cleaned with ethanol and toluene. The photosensibilized formulation containing ESBO, Iod and curcumin (100 μL) were then deposited and spin-coated on the dried stainless steel substrates. Both sides of stainless steel plate were irradiated at a distance of 6 cm at room temperature by means of a Lightningcure LC8-03 lamp from Hamamatsu, equipped with a xenon lamp (200 W) coupled with a flexible light guide. The irradiation time was fixed at 600 s per side with an intensity of 30 mW cm−2.3.7 Thermo gravimetric analysis (TGA)
10 mg of the coating were introduced into aluminium pans and was analyzed using on a Setaram Setsys Evolution 16 thermobalance by heating the sample from 20 °C to 800 °C with a heating rate of 15 °C min−1, under air.3.8 Fluorescence
The absorption measurements were carried out with a Perkin-Elmer Lambda 2 spectrometer. Steady-state fluorescence spectra were collected from a FluoroMax-4 spectrofluorometer. Emission spectra were spectrally corrected. The interaction rate constants kq between curcumin and Iod were extracted from Stern–Volmer treatment (I0/I = 1 + kqτ0[Iod]; where I0 and I stand for the fluorescence intensity of curcumin without and with the presence of Iod, respectively; τ0 stands for the lifetime of curcumin without Iod).3.9 Phosphorescence
Phosphorescence measurements were performed in ethanol at 77 K. The samples are placed in a 5 mm diameter quartz tube inside a Dewar filled with liquid nitrogen. The phosphorescence lifetimes were measured using a FluoroMax-4 spectrofluorometer, which is also equipped with a Xe-pulsed lamp operating at up to 25 Hz. The phosphorescence decays are obtained according to a time-gated method. The emission is recorded using a control module that includes a gate-and-delay generator that allows the integration of the signal during a specific period after a flash (delay) and for a predetermined time window. The total signal is accumulated for a large number of exciting pulses.3.10 Fluorescence microscopy
Inverted microscope IX73 from Olympus equipped with a 75 W Xe lamp housing. The excitation and emission light is filtered with a fluorescence mirror unit (U-FUN from Olympus) associating a band pass filter centered at 365 nm (BP360-370), dichroic mirror (DM410) and long pass filter (BA420IF).3.11 Redox potentials
The oxidation potential (Eox vs. SCE) of the studied curcumin was measured in acetonitrile by cyclic voltammetry with tetrabutylammonium hexafluorophosphate (0.1 M) as supporting electrolyte (Voltalab 6 Radiometer). The working electrode was a platinium disk and the reference electrode was a saturated calomel electrode (SCE). The free energy change ΔG for an electron transfer between the studied compounds and Iod can be calculated for the classical Rehm–Weller equation (eqn (3), where Eox, Ered, Es (or ET), C are the oxidation potential of the studied compounds, the reduction potential of Iod, the excited (or triplet) state energy of the studied compounds, and the electrostatic interaction energy for the initiatlly formed ion pair, generally considered as negligible in polar solvents:52| ΔG = Eox − Ered − Es (or ET) + C | (3) |
3.12 Nanoindentation and scratch tests
Nanoindentation and scratch tests were carried out on the coatings (deposited on glass and steel substrates) with a Nano Indenter (Agilent Technologies G200) using a Berkovich tip (Micro Star Technologies). Twenty nanoindentation tests per sample were performed. Samples were loaded and unloaded at constant strain rate (0.05 s−1) using the Continuous Stiffness Measurement (CSM) method until an indentation depth of 1 μm. The unloading stage was performed after a hold load plateau of 300 s in order to exhibit the viscous behaviour. Twenty scratch tests were performed; face forward, with an increasing load from 0.1 to 100 mN for a scratching distance of 500 μm. The distance between two scratches was fixed to 500 μm.3.13 Antibacterial property of the coatings
Initial adhesion assays were performed using two strains of bacteria, namely E. coli ATCC25922 and S. aureus ATCC6538 on the curcumin derivative-coatings. Prior to in vitro antibacterial tests, the bacterial strains were grown aerobically overnight in Luria–Bertani broth at 37 °C under stirring. Overnight cultures of E. coli and S. aureus grown in Luria–Bertani (LB) broth were diluted to an optical density (OD 600 nm) of 0.05 in sterile LB broth. At this point, the inox supports and inox substrates with coatings (1.5 cm × 1.5 cm) were immersed in the culture; the corresponding vials were placed on a slantwise rotating wheel to avoid the sedimentation of bacteria, incubated for different times (2 h, 6 h, 24 h and 48 h) and shaken at 150 rpm to allow initial adhesion to occur (INFORS AG-CH 4103, Bottmingen-Basel, Switzerland). During the incubation time, some samples are illuminated with 4 lamps (intensity = 170 μmol m−2 s−1). The emission spectrum is given in Fig. S2.† Following adhesion, the samples were rinsed seven times with sterile saline solution (NaCl, 0.9% w/v) to remove any non-adherent cells.Colonized native and treated samples were then transferred to 2 ml sterile saline (solution A) and vortexed vigorously for 30 s. The samples were then transferred to 2 ml sterile saline (solution B) and sonicated in a Branson 2200 sonicator for 3 min. Samples were transferred once more to 2 ml sterile saline (solution C) and vortexed vigorously for 30 s. Suspensions A, B and C were pooled, serially diluted and plated on PCA medium for viable counting. The cells removed during these three phases represent the loosely attached biofilm population. A 100 μL volume of the detached viable bacteria solution was introduced onto the surface of a Plat Count agar plate. The process was repeated through a succession of 24 pre-dried substrates. Finally, the total bacterial adhesion was determined by a counting of the CFUs, after overnight statically incubation of the agar plates at 37° C. Each experiment was done four times. Levels of adhesion were given as numbers of cells per square centimeter.
3.14 Antibacterial property of curcumin in solution
The reference solution (bacteria/DMSO) and the curcumin solution in DMSO (160 μM) containing bacteria were placed on a slantwise rotating wheel to avoid sedimentation of bacteria, incubated for 2 h, 6 h, 24 h and 48 h at room temperature under visible illumination or not. The volume of DMSO corresponds to 3% v/v of the total bacteria solution. The process is the same as described in the previous paragraph. The suspensions were pooled and serially diluted. A 100 μL volume of the viable bacteria solution was introduced onto the surface of a Plate Count agar plate.Finally, the number of viable bacteria was determined by a counting of the CFUs, after overnight statically incubation of the agar plates at 37 °C. Each experiment was done four times.
3.15 Statistical analysis
All values corresponding to the anti-adherence property of E. coli and S. aureus are expressed as mean ± standard deviation. Statistical analysis was performed using Student's t-test for the calculation of significance level of the data. Differences were considered statistically significant at p < 0.05. Ten samples per group were evaluated.4. Conclusions
This investigation demonstrates for the first time, the ability of curcumin to be used both as a photosensitizer and an antibacterial agent (even incorporated in a coating) against E. coli and S. aureus. The resulting coatings derived from the epoxidized soybean oil which have been synthesized under visible light illumination, display a good adherence properties on stainless steel substrates and a high thermal stability by 375 °C.The synthesized coatings containing curcumin led under visible light activation to a reduction of the S. aureus and E. coli growth by 99% and 95% respectively, after 48 h of incubation. This new coating could successfully be used to avoid bacteria proliferation and deposited on paramedical devices like disposable clamp or disposable scalpel which have only one-time use for few hours.
Acknowledgements
We would like to thank CNRS institute and University of Paris-Est Creteil for financial support and Léon Pereira for the cutting process of stainless steel substrates.References
- R. M. Klevens, J. R. Edwards, J. C. L. Richards, T. C. Horan, R. P. Gaynes, D. A. Pollock and D. M. Cardo, Public Health Rep., 2007, 122, 160–166 Search PubMed.
- M. F. Sampredo and R. Patel, Infect. Dis. Clin., 2007, 21, 785–819 CrossRef PubMed.
- B. Hota, Clin. Infect. Dis., 2004, 39, 1182–1189 CrossRef PubMed.
- I. P. Parkin and R. G. Palgrave, J. Mater. Chem., 2005, 15, 1689–1695 RSC.
- J. Genzer and K. Efimenko, Biofouling, 2006, 22, 339–360 CrossRef CAS PubMed.
- G. Cheng, Z. Zhang, S. F. Chen, J. D. Bryers and S. Y. Jiang, Biomaterials, 2007, 28, 4192–4199 CrossRef CAS PubMed.
- G. M. Manecka, J. Labrash, O. Rouxel, P. Dubot, J. Lalevée, S. A. Andaloussi, E. Renard, V. Langlois and D. L. Versace, ACS Sustainable Chem. Eng., 2014, 2, 996–1006 CrossRef CAS.
- R. Poupart, A. Haider, J. Babinot, I. K. Kang, J. P. Malval, J. Lalevée, S. A. Andalloussi, V. Langlois and D. L. Versace, ACS Biomater. Sci. Eng., 2015, 1, 525–538 CrossRef CAS.
- M. Ignatova, K. Starbova, N. Markova, N. Manolova and I. Rashkov, Carbohydr. Res., 2006, 341, 2098–2107 CrossRef CAS PubMed.
- M. Ignatova, Z. Petkova, N. Manolova, N. Markova and I. Rashkov, Macromol. Biosci., 2012, 12, 104–115 CrossRef CAS PubMed.
- S. E. Habnouni, V. Darcos, X. Garric, J.-P. Lavigne, B. Nottelet and J. Coudane, Adv. Funct. Mater., 2011, 21, 3321–3330 CrossRef PubMed.
- C. Lorenzini, A. Haider, I.-K. Kang, M. Sangermano, S. Abbad-Andalloussi, P.-E. Mazeran, J. Lalevée, E. Renard, V. Langlois and D.-L. Versace, Biomacromolecules, 2015, 16, 683–694 CrossRef CAS PubMed.
- T. T. Ruckh, R. A. Oldinski, D. A. Carroll, K. Mikhova, J. D. Bryers and K. C. Popat, J. Mater. Sci.: Mater. Med., 2012, 23, 1411–1420 CrossRef CAS PubMed.
- J. Lu, M. A. Hill, M. Hood, J. D. F. Greeson, J. R. Horton, P. E. Orndorff, A. S. Herndon and A. E. Tonelli, J. Appl. Polym. Sci., 2001, 82, 300–309 CrossRef CAS PubMed.
- D. Chung, S. E. Papadakis and K. L. Yam, Int. J. Food Sci. Technol., 2003, 38, 165–169 CrossRef CAS.
- M. L. W. Knetsch and L. H. Koole, Polymers, 2011, 3, 340–366 CrossRef CAS PubMed.
- Z. Markovic, B. Todorovic-Markovic, D. Kleut, N. Nikolic, S. Vranjes-Djuric, M. Misirkic, L. Vucicevic, K. Janjetovic, A. Isakovic, L. Harhaji, B. Babic-Stojic, M. Dramicanin and V. Trajkovic, Biomaterials, 2007, 28, 5437–5448 CrossRef CAS PubMed.
- M. J. Niedre, A. J. Secord, M. S. Patterson and B. C. Wilson, Cancer Res., 2003, 63, 7986–7994 CAS.
- X. Ragas, L. P. Cooper, J. H. White, S. Nonell and C. Flors, ChemPhysChem, 2011, 12, 161–165 CrossRef CAS PubMed.
- X. Ragas, M. Agut and S. Nonell, Free Radical Biol. Med., 2010, 49, 770–776 CrossRef CAS PubMed.
- R. Cahan, R. Schwartz, Y. Langzam and Y. Nitzan, Photochem. Photobiol., 2011, 87, 1379–1386 CrossRef CAS PubMed.
- V. Decraene, J. Pratten and M. Wilson, Curr. Microbiol., 2008, 57, 269–273 CrossRef CAS PubMed.
- V. Decraene, J. Pratten and M. Wilson, Infect. Control Hosp. Epidemiol., 2008, 29, 1181–1184 CrossRef PubMed.
- S. Ismail, S. Perni, J. Pratten, I. Parkin and M. Wilson, Infect. Control Hosp. Epidemiol., 2011, 32, 1130–1132 CrossRef PubMed.
- V. Decraene, D. Ready, J. Pratten and M. Wilson, J. Gen. Appl. Microbiol., 2008, 54, 195–203 CrossRef CAS.
- P. G. Calzavara-Pinton, M. Venturini and R. Sala, J. Photochem. Photobiol., B, 2005, 78, 1–6 CrossRef CAS PubMed.
- T. Maisch, C. Bosl, R. M. Szeimies, N. Lehn and C. Abels, Antimicrob. Agents Chemother., 2005, 49, 1542–1552 CrossRef CAS PubMed.
- R. F. Donnelly, P. A. McCarron and M. M. Tunney, Microbiol. Res., 2008, 163, 1–12 CrossRef CAS PubMed.
- T. A. Dahl, W. R. Midden and P. E. Hartman, J. Bacteriol., 1989, 171, 2188–2194 CAS.
- G. Jori, J. Environ. Pathol., Toxicol. Oncol., 2006, 25, 505–519 CrossRef.
- M. Wilson, Infect. Control Hosp. Epidemiol., 2003, 24, 782–784 Search PubMed.
- A. Felgentrager, T. Maisch, A. Spath, J. A. Schroder and W. Baumler, Phys. Chem. Chem. Phys., 2014, 16, 20598–20607 RSC.
- S. Yano, S. Hirohara, M. Obata, Y. Hagiya, S.-I. Ogura, A. Ikeda, H. Kataoka, M. Tanaka and T. Joh, J. Photochem. Photobiol., C, 2011, 12, 46–67 CrossRef CAS PubMed.
- K. W. Oh, H.-M. Choi, J. M. Kim, J. H. Park and I. S. Park, Text. Res. J., 2014, 84, 808 CrossRef PubMed.
- M. N. Usacheva, M. C. Teichert and M. A. Biel, Lasers Surg. Med., 2001, 29, 165–173 CrossRef CAS PubMed.
- G. E. Cohn and H. Y. Tseng, Photochem. Photobiol., 1977, 26, 465–474 CrossRef CAS PubMed.
- F. Freire, A. Costa, C. Pereira, M. Beltrame Junior, J. Junqueira and A. Jorge, Laser. Med. Sci., 2014, 29, 949–955 CrossRef PubMed.
- P. V. Araujo, K. I. Teixeira, L. D. Lanza, M. E. Cortes and L. T. Poletto, Acta Odontol. Latinoam., 2009, 22, 93–97 Search PubMed.
- Y. Guo, S. Rogelj and P. Zhang, Nanotechnology, 2010, 21, 065102 CrossRef PubMed.
- S. Dutta, S. Padhye, K. I. Priyadarsini and C. Newton, Bioorg. Med. Chem. Lett., 2005, 15, 2738–2744 CrossRef CAS PubMed.
- B. B. Aggarwal, C. Sundaram, N. Malani and H. Ichikawa, Adv. Exp. Med. Biol., 2007, 595, 1–75 CrossRef.
- G. Bar-Sela, R. Epelbaum and M. Schaffer, Curr. Med. Chem., 2010, 17, 190–197 CrossRef CAS.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Mol. Pharmaceutics, 2007, 4, 807–818 CrossRef CAS PubMed.
- S. M. Khopde, K. I. Priyadarsini, D. K. Palit and T. Mukherjee, Photochem. Photobiol., 2000, 72, 625–631 CrossRef CAS.
- K. I. Priyadarsini, J. Photochem. Photobiol., C, 2009, 10, 81–95 CrossRef CAS PubMed.
- H.-J. Kim, D.-J. Kim, S. N. Karthick, K. V. Hemalatha, C. J. Raj, S. Ok and Y. Choe, Int. J. Electrochem. Sci., 2013, 8, 8320–8329 CAS.
- J. Zhao, J. Lalevée, H. Lu, R. MacQueen, S. H. Kable, T. W. Schmidt, M. H. Stenzel and P. Xiao, Polym. Chem., 2015, 6, 5053–5061 RSC.
- J. V. Crivello and U. Bulut, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5217–5231 CrossRef CAS PubMed.
- J. V. Crivello and U. Bulut, Macromol. Symp., 2006, 240, 1–11 CrossRef CAS PubMed.
- M. Jin, J. Xie, J.-P. Malval, A. Spangenberg, O. Soppera, D.-L. Versace, T. Leclerc, H. Pan, D. Wan, H. Pu, P. Baldeck, O. Poizat and S. Knopf, J. Mater. Chem. C, 2014, 2, 7201–7215 RSC.
- G. Pohlers, J. C. Scaiano and R. Sinta, Chem. Mater., 1997, 9, 3222–3230 CrossRef CAS.
- D. Rehm and A. Weller, Isr. J. Chem., 1970, 8, 259–271 CrossRef CAS PubMed.
- J.-P. Fouassier and J. Lalevée, Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency, Wiley-VCH Verlag GmbH and Co KGaA, Weinheim, 2012 Search PubMed.
- T. A. Dahl, W. R. Midden and P. E. Hartman, Photochem. Photobiol., 1987, 46, 345–352 CrossRef CAS PubMed.
- D. Bellus, H. Lind and J. F. Wyatt, J. Chem. Soc., Chem. Commun., 1972, 1199–1200 RSC.
- G. Gryn’ova, K. U. Ingold and M. L. Coote, J. Am. Chem. Soc., 2012, 134, 12979–12988 CrossRef PubMed.
- A. Gomes, E. Fernandes and J. L. F. C. Lima, J. Biochem. Biophys. Methods, 2005, 65, 45–80 CrossRef CAS PubMed.
- M. C. DeRosa and R. J. Crutchley, Coord. Chem. Rev., 2002, 233–234, 351–357 CrossRef CAS.
- D. J. Balazs, K. Triandafillu, P. Wood, Y. Chevolot, C. v. Delden, H. Harms, C. Hollensteind and H. J. Mathieu, Biomaterials, 2004, 25, 2139–2151 CrossRef CAS PubMed.
- P. J. Eginton, H. Gibson, J. Holah, P. S. Handley and P. Gilbert, J. Ind. Microbiol., 1995, 15, 305–310 CrossRef CAS.
- W. Zhou, S. M. Kuebler, D. Carrig, J. W. Perry and S. R. Marder, J. Am. Chem. Soc., 2002, 124, 1897–1901 CrossRef CAS PubMed.
- M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, Handbook of Photochemistry, CRC Press, Boca Raton, 3rd edn, 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Evolution of the absorbance of 1,3-diphenylisobenzofuran (DBF) and curcumin alone at 480 nm in acetonitrile under visible light illumination and emission spectrum of the 4 lamps used during the different incubation times. See DOI: 10.1039/c5ra19499g |
| This journal is © The Royal Society of Chemistry 2015 |