DOI:
10.1039/C5RA16259A
(Paper)
RSC Adv., 2015,
5, 85202-85213
ZrO2 nanoparticles as a reusable solid dual acid–base catalyst for facile one-pot synthesis of multi-functionalized spirooxindole derivatives under solvent free condition†
Received
12th August 2015
, Accepted 29th September 2015
First published on 30th September 2015
Abstract
A two-step one-pot protocol for the facile synthesis of biologically important spirooxindole derivatives such as spiro[4H-pyran-3,3′-oxindoles] and spiro[indoline-3,4′(1H′)-pyrano-[2,3-c]pyrazol-2-ones has been developed. In this method ZrO2 nanoparticles have been utilized as a reusable solid dual acid–base catalyst to get quick access to the multi-functionalized spirooxindole derivatives under solvent free condition at room temperature. The main advantages of this method are the operational simplicity, reduced reaction time, elimination of solvents, high yield of the products, convenient work up procedure and employment of nontoxic and recyclable ZrO2 nano catalyst. All these factors make the present method economical, green and sustainable.
Introduction
The practices of green chemistry hold the key to build an environmentally sustainable society.1 In recent time, metal based nanoparticles (NPs) in the form of nano catalysts have emerged as viable alternatives to conventional materials in various fields of chemistry and attracted the interest of chemists in exploration new green synthetic pathways.2 The metal based NPs are known to be promising materials for heterogeneous catalysts in a variety of organic transformations.3 The surface of ZrO2 NPs is reported to contain active hydroxyl groups, oxyanions and Zr4+ ions which can act as dual acid–base catalyst.4 This interesting property of ZrO2 NPs has not been explored as yet under solvent free condition, especially in important multicomponent reactions for synthesis of bio-active building blocks. In continuation of our research work on the synthesis of isatin based heterocycles5 and development of green synthetic methodologies,5b,5c,6 herein, we explore the potential of nontoxic ZrO2 NPs as heterogeneous catalyst in multicomponent reactions. Moreover the study will provide valuable insights into the mechanism of the reaction and the mode of catalytic activity of ZrO2 NPs which will help in expanding the scope of the catalyst in new synthetic design.
The indole ring system is probably the most ubiquitous heterocyclic moiety found in a large number of bioactive natural products and medicinal agents.7 The spirooxindole derivatives also occupy a special place in organic and medicinal chemistry since they exhibit diverse biological and pharmacological activities. To name a few, spirotryprostatin A and B, for instance, are well-known microtubule assembly inhibitors (Fig. 1).8 Isopteropodine and pteropodine are found to act as muscarinic M1 and serotonin receptor modulators (Fig. 1).9 MK-0677 is bioactive as nonpeptidyl growth-hormone secretagogues.10 In light of their unique structural features along with the important biological activities, spirooxindole derivatives have drawn considerable attention to synthetic chemists for their preparation.11 While a number of methods for the synthesis of spirooxindole derivatives have been documented in the literature, the development of new routes for efficient construction of the spirooxindole core with diverse functionalized structures remains desired. Therefore we wish to report herein a facile one-pot synthesis of two series of spirooxindole derivatives such as spiro[4H-pyran-3,3′-oxindoles] (4) and spiro[indoline-3,4′(1H′)-pyrano-[2,3-c]pyrazol-2-ones (7) through the condensation of isatin (1) and malononitrile (2) with cyclohexane-1,3-diones (3) and ethylacetoacetate (5) and hydrazines (6) in presence of ZrO2 nano catalyst under solvent-free condition at room temperature (Scheme 1).
 |
| | Fig. 1 Some spirooxindole based biologically active natural products. | |
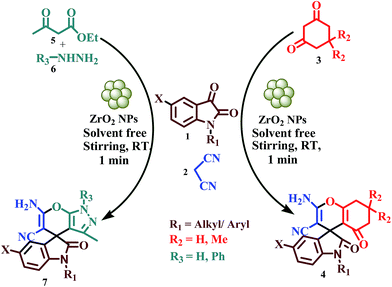 |
| | Scheme 1 Synthesis of spiro[4H-pyran-3,3′-oxindoles] (4) and spiro[indoline-3,4′(1H′)-pyrano-[2,3-c]pyrazol-2-one derivatives (7). | |
Results and discussion
During the optimization studies towards the development of ZrO2 as nano catalyst for one-pot synthesis of spirooxindole derivatives, two different set of reactions such as condensation of isatin (1), malononitrile (2) and dimedone (3) and condensation of isatin (1), malononitrile (2), ethylacetoacetate (5) and hydrazine (6) were chosen as model reactions (Scheme 2). In these processes various parameters such as the effect of catalysts, solid supports and solvents at different experimental conditions have been examined (Table 1). In the initial investigation, two reaction mixtures, taking the reactants 1.0 mmol each in ethanol, were stirred at room temperature. Under this conditions the condensation of isatin (1), malononitrile (2) and dimedone (3) afforded the product 4a in ∼70% yield and condensation of isatin (1), malononitrile (2), ethylacetoacetate (5) and hydrazine (6) furnished the product 7a in ∼74% yield within 10 minutes (Scheme 2; Table 1, entry 1). These reactions were found to be two-step one-pot processes where the reactants 1 and 2 were added initially and then after two minutes the remaining reactants (3 or 5 and 6) were added sequentially. When the same reactions were carried out in water medium the product 4a and 7a were formed in ∼65 and 62% yield respectively (Table 1, entry 2). The low yield of the products was due to low solubility of the reactants in water medium. Employment of 10 mol% Et3N or piperidine as catalyst enhanced the products formation slightly in EtOH medium at room temperature (Table 1, entries 3 and 4). Addition of various acid catalysts (20 mol%) such as lactic acid, formic acid and p-TSA in EtOH medium not only showed insignificant reaction catalysis but also gradual lowering of products yield with increase of acidity (Table 1, entries 5–7). The presence of organocatalyst L-proline (20 mol%) in EtOH medium also could not enhance the products yield significantly at room temperature (Table 1, entry 8). Surprisingly when ZrO2 NPs (20 mol%) were used as catalyst in EtOH and water medium substantial catalysis was observed in both cases producing 4a and 7a in good yields within 5 minutes (Table 1, entries 9 and 10).
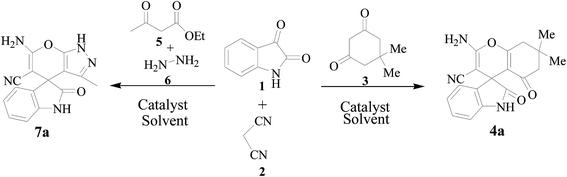 |
| | Scheme 2 Optimization of the reaction conditions. | |
Table 1 Optimization of reaction conditions for the synthesis of 4a and 7aa
| Entry |
Solvent (5.0 mL) |
Catalyst |
Catalyst load |
Time (min) |
Yieldb (%) |
| 4a |
7a |
| All the reactions were carried out at room temperature taking the reactants in 1.0 mmol each. Isolated yields. |
| 1 |
EtOH |
— |
— |
10 |
70 |
74 |
| 2 |
H2O |
— |
— |
15 |
65 |
62 |
| 3 |
EtOH |
Et3N |
10 mol% |
10 |
74 |
76 |
| 4 |
EtOH |
Piperidine |
10 mol% |
10 |
78 |
79 |
| 5 |
EtOH |
Lactic acid |
20 mol% |
15 |
76 |
77 |
| 6 |
EtOH |
Formic acid |
20 mol% |
15 |
70 |
71 |
| 7 |
EtOH |
p-TSA |
20 mol% |
15 |
68 |
69 |
| 8 |
EtOH |
L-Proline |
20 mol% |
15 |
72 |
73 |
| 9 |
EtOH |
ZrO2 NPs |
20 mol% |
5 |
87 |
88 |
| 10 |
H2O |
ZrO2 NPs |
20 mol% |
5 |
86 |
85 |
| 11 |
— |
— |
— |
20 |
— |
— |
| 12 |
— |
p-TSA |
20 mol% |
15 |
68 |
67 |
| 13 |
— |
SSA |
200 mg |
5 |
60 |
61 |
| 14 |
— |
PEG-OSO3H |
200 mg |
15 |
65 |
63 |
| 15 |
— |
Melamine sulfonic acid (MSA) |
200 mg |
10 |
63 |
67 |
| 16 |
— |
ZrO2 NPs |
20 mol% |
1 |
86 |
91 |
| 17 |
— |
ZrO2 NPs |
10 mol% |
1 |
84 |
90 |
| 18 |
— |
ZrO2 NPs |
5 mol% |
1 |
84 |
90 |
In quest of greener conditions, subsequently both the reactions were performed under solvent free condition without using any catalyst. In this condition no product was formed even after stirring the reaction mixtures for 20 minutes at room temperature (Table 1, entry 11). Then the feasibility of the reactions under solvent free condition was examined in presence of various strong solid acid catalysts such as p-TSA (20 mol%), SSA (200 mg), PEG-OSO3H (200 mg) and melamine sulfonic acid (250 mg) (Table 1, entries 12–15). To our delight, we observed that the selected solid acids can catalyze the condensation reactions significantly under solvent free condition at room temperature. In search of better yield, reduced reaction time and cleaner reaction profile, next 20 mol% ZrO2 NPs was employed as catalyst for the reactions under solvent free condition. Gratifyingly, in this case the product 4a and 7a were formed in high yield, ∼86 and 91% respectively on stirring the reaction mixtures for 5 minutes at room temperature (Table 1, entry 16). Then we carried out the optimization study to examine the influence of the stoichiometry of ZrO2 NPs (20–5 mol%) under solvent free condition for the best yield of product 4a and 7a (Table 1, entries 16–18). The study revealed that the best yield of the product 4a and 7a can be obtained when the reactants are allowed to react on the solid surface of ZrO2 NPs (5.0 mol%) at room temperature just for 1 minute under solvent-free condition (Table 1, entry 18). It is envisioned that, the adsorption of the reactant molecules on the large surface area of ZrO2 NPs increases the local concentration around the active sites and accelerates the reaction rate remarkably.
With the optimized condition in hand, next the scope and generality of this protocol was assessed by employing various isatins, cyclohexane-1,3-diones and hydrazines (Scheme 1). The results show that the optimized methodology (Table 1, entry 18) tolerates a wide spectrum of substrates to produce multi-functionalized spirooxindole derivatives 4a–s and 7a–w in good to excellent yields (Table 2 and 3). The formation of the final products 4 and 7 was confirmed by IR, 1H NMR, 13C NMR spectroscopy, elemental analysis and also by matching the melting points of some of the compounds with the reported values.11a–i The determination of the X-ray crystal structures of 4n and 7e further corroborates the products formation (Fig. 2 and 3).
Table 2 Library synthesis of spiro[4H-pyran-3,3′-oxindoles] compounds 4a–sa
| Isolated yields. |
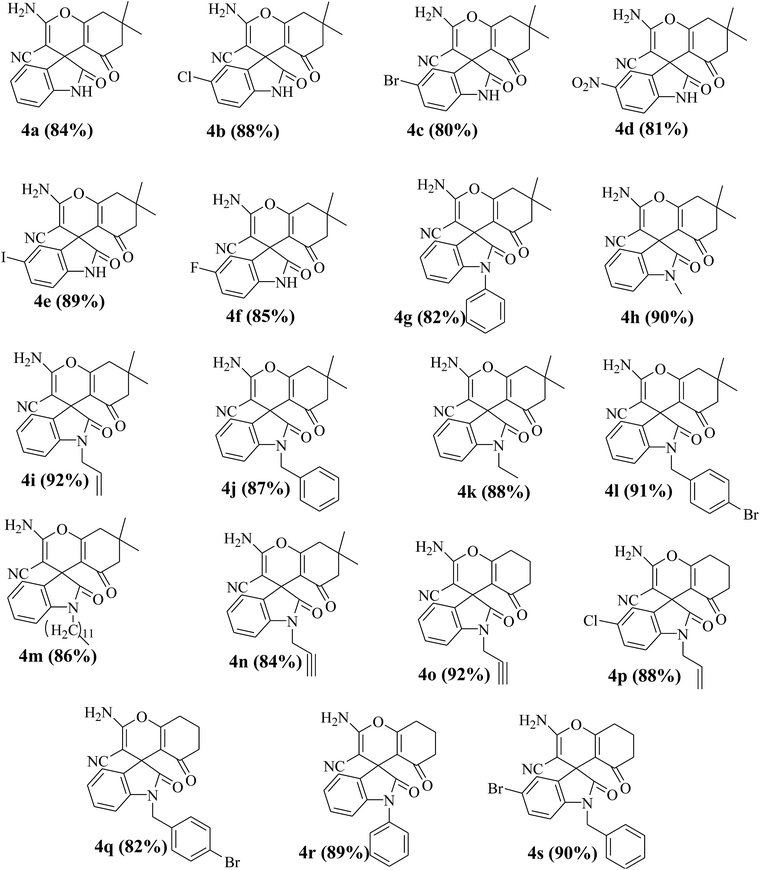 |
Table 3 Library synthesis of spiro[indoline-3,4′(1H′)-pyrano-[2,3-c]pyrazol-2-one derivatives 7a–wa
| Isolated yields. |
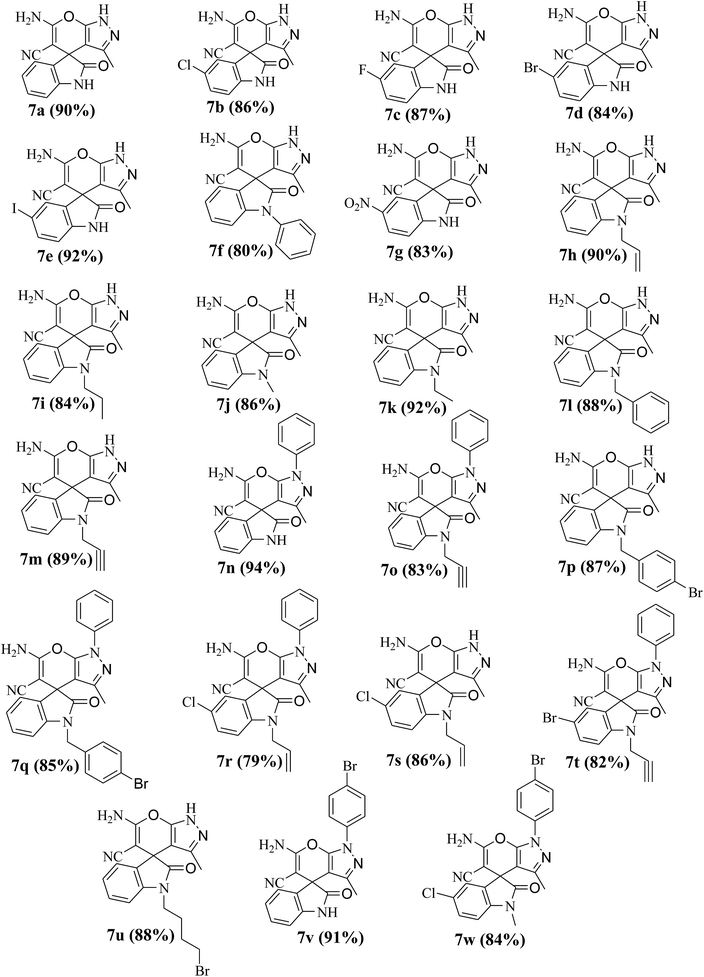 |
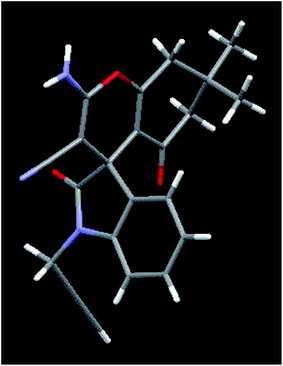 |
| | Fig. 2 Crystal structure of compound 4n. Color code: red, oxygen; blue, nitrogen; grey, carbon; white, hydrogen (CCDC 1409892).† | |
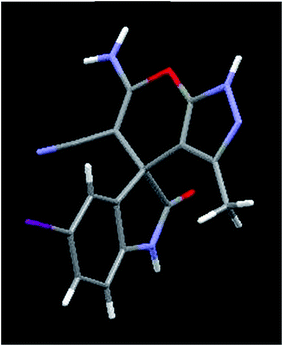 |
| | Fig. 3 Crystal structure of compound 7e. Color code: red, oxygen; blue, nitrogen; grey, carbon; white, hydrogen; purple, iodine (CCDC 1411963).† | |
The morphology of the prepared ZrO2 NPs has been determined from scanning electron microscopic (SEM) study. The SEM images indicate the formation of spherical and nearly uniform sized ZrO2 NPs (Fig. 4a and b). The morphology and uniformity of particle size of ZrO2 NPs were further characterized by high-resolution transmission electron microscopic (HRTEM) images, before (Fig. 4c) and after five times applications (Fig. 4d) at an accelerating voltage of 200 kV. Elemental analyses of the as-synthesized ZrO2 NPs were performed at EDX equipped onto HRTEM and found to be quite consistent (Fig. 5). Further the characterization of as-synthesized ZrO2 NPs has been carried out by powder XRD study. The XRD patterns of freshly prepared ZrO2 NPs and after five times reused ZrO2 NPs are displayed in Fig. 6. The powder X-ray diffraction pattern of the catalyst indicates the formation of tetragonal ZrO2 (tZrO2) NPs.12 No characteristics changes in the peaks are observed after 5 times reuse of the ZrO2 NPs which suggests that the arrangement of the catalyst remains unchanged after the reusability test.13 Further characterization of ZrO2 NPs catalyst was confirmed by FT-IR and UV-visible studies (ESI†).
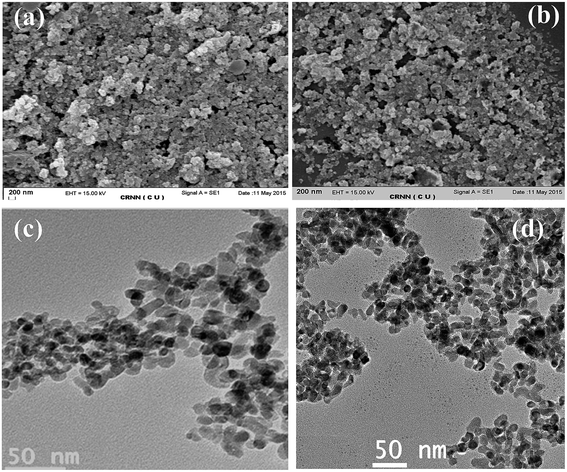 |
| | Fig. 4 SEM images of ZrO2 NPs (a) before reaction and (b) after 5 times applications in reaction. HRTEM images of ZrO2 NPs (c) before reaction and (d) after five times applications. | |
 |
| | Fig. 5 TEM-EDX of ZrO2 NPs. | |
 |
| | Fig. 6 XRD spectra of (A) freshly prepared ZrO2 NPs and (B) reused ZrO2 NPs after 5th cycle. | |
Mechanism
A plausible mechanism for the formation of spirooxindoles 4 and 7 using ZrO2 NPs under solvent free condition has been described in Scheme 3. The surface of ZrO2 NPs is decorated with active hydroxyl, oxide and Zr4+ ions4 which can act as dual acid–base catalyst for the condensation reactions. On adsorption of the reactant molecules on the solid surface of ZrO2 NPs the local concentration of the reactants around the active sites increases significantly causing acceleration of the reaction rate. Initially, malononitrile (2) condenses with isatins (1) via Knoevenagel condensation to form a common intermediate (I) promoted by acid–base catalysis of ZrO2 NPs as shown in Scheme 3. Then the intermediate I undergoes Michael type condensation with the pyrazole intermediate (II) derived in situ from ethylacetoacetate (5) and hydrazine (6) to form the intermediate III (Scheme 3a). Subsequently the acidic and basic sites of ZrO2 NPs assist the intermediate III to undergo intra-molecular electrophilic cyclization followed by tautomerization, to afford the desired spirooxindoles 7. Similarly the intermediate I undergoes Michael type addition with the enol form of cyclohexane-1,3-diones (IV) to furnish spirooxindoles 4 through the formation of intermediate V (Scheme 3b).
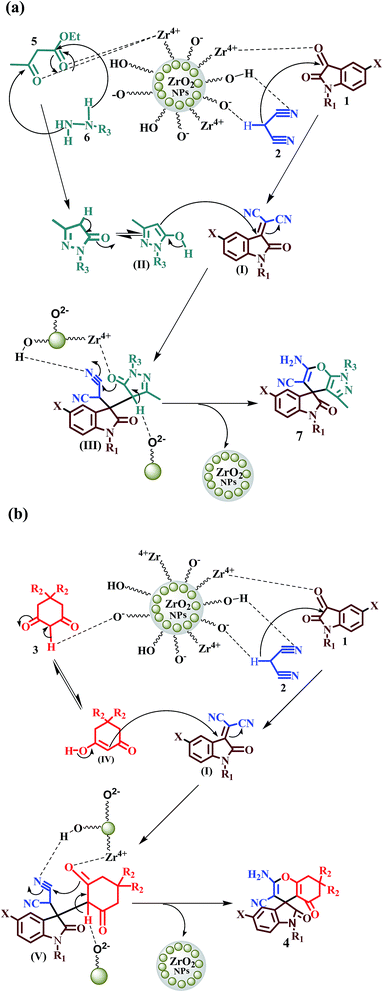 |
| | Scheme 3 Plausible mechanism for the formation of (a) spirooxindoles 7 and (b) spirooxindoles 4. | |
The reusability test of ZrO2 NPs was carried out separately for the synthesis of 4a and 7a under the optimized reaction conditions (Table 1, entry 18). At the end of the reactions, the reaction mixtures were ultrasonicated with ethylacetate to extract the products 4a and 7a from the surface of the solid support and the solid ZrO2 nanoparticles were separated easily from the reaction mixture by simple decantation method. Then ZrO2 NPs were washed three times with ethylacetate (3 × 5 mL), dried at room temperature under vacuum to eliminate residual solvents and used for the next cycle. The condensation reactions for the synthesis of 4a and 7a were performed with the recovered catalyst upto five times. The result shows that there is indeed substantial retention of initial catalytic activity of ZrO2 NPs even after repeated applications (Fig. 7).
 |
| | Fig. 7 Reusability of ZrO2 NPs for the formation of 4a (red bar) and 7a (light green bar). | |
Conclusions
In summary, we have developed an efficient, eco-friendly and sustainable methodology to get quick access to several multi-functionalized spirooxindole derivatives such as spiro[4H-pyran-3,3′-oxindoles] and spiro[indoline-3,4′(1H′)-pyrano-[2,3-c]pyrazol-2-ones under solvent-free condition at room temperature. The method involves two-step one-pot condensation of isatins, malononitrile, cyclohexane 1,3-diones and pyrazoles in presence of ZrO2 nanoparticles as a solid dual acid–base catalyst. To the best our knowledge this is the first example where ZrO2 nanoparticles have been exploited as a solid supported dual acid–base catalyst in important condensation reactions for synthesis of bio-active building blocks. The significant advantages of this methodology are the use of solvent-free reaction conditions, employment of simple and easily available starting materials and reagents, operational simplicity of the reaction and the use of a nontoxic and recyclable catalyst. All these factors make the present method economical, green and sustainable.
Experimental section
General methods
Solvents and chemicals were purchased from commercial suppliers and used without further purification. Catalyst and starting materials were prepared according to reported procedure. Melting points were measured in open capillary tubes and were uncorrected. JASCO FT/IR-6300 spectrophotometer was used for IR spectra. 1H (300 MHz) and 13C NMR (75 MHz) spectra were performed on Bruker instrument (300 MHz) in DMSO-d6. Elemental analyses (C, H and N) were recorded using Perkin-Elmer 240C elemental analyzer. The X-ray diffraction data for crystallized compounds were collected with MoKα radiation at 296 K using the Bruker APEX-II CCD System. The morphological analysis of the resultant nanoparticles was confirmed by HRTEM, using a HRTEM, JEOL JEM 2010 at an accelerating voltage of 200 kV and fitted with a CCD camera. The crystallinity of synthesized ZrO2 nanoparticles was determined and confirmed by XRD analysis. The diffractogram was documented from PANalytical, XPERT-PRO diffractrometer using Cuα (λ = 1.54060) as X-ray source. Hitachi S-3400N Scanning Electron Microscope was used for SEM.
General synthetic procedure for the preparation of compounds 4
An equimolar mixture of isatins (1) (1.0 mmol) and malononitrile (2) (1.0 mmol) was dissolved in minimum quantity of chloroform (3 mL) in a 100 mL round bottom flask and to this mixture ZrO2 NPs (5.0 mol%) were added and mixed thoroughly. Then the solvent was removed from the mixture under vacuum to get a semi liquid mixture. Subsequently cyclohexane-1,3-diones (1.0 mmol) were added and the resulting mixture was stirred at room temperature for 1–2 minutes. The progress of the reaction was monitored by thin layer chromatography (TLC). At the end of the reaction, ethylacetate was added to the reaction mixture and the product was extracted from the solid support by ultrasonication. The ZrO2 NPs were separated by decantation method and the separated organic phase was collected in another round bottom flask. Then the organic layer was evaporated to get the crude mass. Pure products 4 were obtained from the isolated crude mass through column chromatography on silica gel using ethylacetate/hexane (∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent.
1) as the eluent.
General synthetic procedure for the preparation of compounds 7
To an equimolar mixture of isatins (1) (1.0 mmol) and malononitrile (2) (1.0 mmol) in a 100 mL round bottom flask, ZrO2 NPs (5 mol%) were added and mixed thoroughly. Then to this semi liquid mixture hydrazines (1.0 mmol) and ethylacetoacetate (1.0 mmol) were added sequentially and the resulting mixture was stirred at room temperature for 1–2 minutes. The progress of the reaction was monitored by thin layer chromatography (TLC). At the end of the reaction, ethylacetate was added to the reaction mixture and the product was extracted from the solid support by ultrasonication. The ZrO2 NPs were separated by decantation method and the separated organic phase was collected in another round bottom flask. Then the organic layer was evaporated to get the crude mass. Pure products 7 were obtained from isolated crude mass through column chromatography on silica gel using ethylacetate/hexane (∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent.
1) as the eluent.
Recycle procedure of catalyst ZrO2 NPs
At the end of the reactions for the preparation of 4a and 7a as described above, the reaction mixtures were ultrasonicated with ethylacetate to extract the products 4a and 7a from the surface of the solid support and the solid ZrO2 NPs were separated easily from the reaction mixture by simple decantation method. Then ZrO2 NPs were washed three times with ethylacetate (3 × 5 mL), dried at room temperature under vacuum to eliminate residual solvents and used for the next cycle. The reactions for the synthesis of 4a and 7a were performed upto five times with the catalyst ZrO2 NPs recovered after each cycle.
Characterization data of 4a–s
4a. White amorphous solid (yield 84%); mp: 288–292 °C. IR (KBr) 3389, 3299, 2950, 2195, 1743, 1165, 1648, 1482 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 10.24 (s, 1H), 7.11 (s, 2H, NH2), 7.07–6.98 (m, 1H), 6.87–6.62 (m, 3H), 2.39 (s, 2H), 2.09–1.95 (m, 2H), 0.91 (s, 3H), 0.88 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 195.3, 178.4, 164.5, 159.2, 142.5, 134.8, 128.6, 123.4, 122.1, 117.7, 111.2, 109.6, 58.0, 50.4, 47.2, 32.3, 28.0, 27.5; C19H17N3O3 (335.35): calcd C 68.05, H 5.11, N 12.53%; found C 67.80, H 5.05, N 12.38%.
4b. White amorphous solid (yield 88%); mp: 296–298 °C. IR (KBr) 3364, 3248, 3172, 2193, 1718, 1680, 1472 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 10.54 (s, 1H), 7.31 (s, 2H, NH2), 7.19–7.16 (dd, J1 = 8.1 Hz, J2 = 1.5 Hz, 1H), 7.09 (s, 1H), 6.79 (d, J = 8.4 Hz, 1H), 2.54 (d, J = 7.5 Hz, 2H), 2.14 (s, 2H), 1.01 (s, 6H); 13C NMR (75 MHz, DMSO-d6) δC 195.5, 178.2, 165.0, 159.3, 141.5, 136.8, 128.5, 126.1, 123.7, 117.6, 111.0, 110.6, 57.2, 50.4, 47.5, 32.4, 27.9, 27.6; C19H16ClN3O3 (369.80): calcd C 61.71, H 4.36, N 11.36%; found C 61.50, H 4.25, N 11.19%.
4c. White amorphous solid (yield 80%); mp: >300 °C. IR (KBr) 3358, 3242, 2189, 1740, 1695, 1483 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 10.54 (s, 1H), 7.31 (bs, 2H), 7.21 (bs, 2H), 6.78 (bs, 1H), 2.56 (d, J = 10.5 Hz, 2H), 2.16 (s, 2H), 1.03 (s, 6H); 13C NMR (75 MHz, DMSO-d6) δC 195.5, 178.1, 165.0, 159.3, 141.9, 137.2, 131.3, 126.4, 117.6, 113.7, 111.6, 110.6, 57.2, 50.4, 47.5, 32.4, 28.0, 27.6; C19H16BrN3O3 (414.25): calcd C 55.09, H 3.89, N 10.14%; found C 54.98, H 3.75, N 10.09%.
4d. Yellow amorphous solid (yield 81%); mp: 296–298 °C. IR (KBr) 3364, 3248, 2185, 1735, 1683, 1539, 1465 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 11.19 (s, 1H), 8.16 (d, J = 6.9 Hz, 1H), 7.97 (s, 1H), 7.47 (s, 2H, NH2), 7.03 (d, J = 8.7 Hz, 1H), 2.70–2.56 (m, 2H), 2.17 (d, J = 5.7 Hz, 2H), 1.03 (s, 6H); 13C NMR (75 MHz, DMSO-d6) δC 195.7, 179.0, 165.6, 159.5, 149.1, 142.8, 135.8, 126.2, 119.2, 117.4, 110.2, 109.8, 56.4, 50.2, 47.4, 32.5, 28.1, 27.4; C19H16N4O5 (380.35): calcd C 60.00, H 4.24, N 14.73%; found C 59.96, H 4.16, N 14.69%.
4e. White amorphous solid (yield 89%); mp: >300 °C. IR (KBr) 3354, 3263, 2187, 1718, 1691, 1480 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 10.41 (s, 1H), 7.37–7.34 (dd, J1 = 7.8 Hz, J2 = 1.2 Hz, 1H), 7.19 (bs, 3H), 6.53 (d, J = 8.1 Hz, 1H), 2.35 (d, J = 8.7 Hz, 2H), 2.03 (s, 2H), 0.89 (s, 6H); 13C NMR (75 MHz, DMSO-d6) δC 195.4, 177.8, 164.9, 159.2, 142.3, 137.4, 137.1, 131.7, 117.5, 112.1, 110.4, 84.7, 57.2, 50.3, 47.2, 32.3, 27.9, 27.5; C19H16IN3O3 (461.25): calcd C 49.47, H 3.50, N 9.11%; found C 49.41, H 3.38, N 9.01%.
4f. White amorphous solid (yield 85%); mp: 292–294 °C. IR (KBr) 3357, 3289, 3147, 2194, 1726, 1659, 1603, 1474 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 10.32 (s, 1H), 7.18 (s, 2H), 6.86–6.81 (m, 2H), 6.66 (m, 1H), 2.42 (s, 2H), 2.03 (s, 2H), 0.90 (s, 6H); 13C NMR (75 MHz, DMSO-d6, F coupled 13C spectra) δC 195.3, 178.4, 164.8, 160.1, 159.2, 157.0, 138.6, 136.5, 117.5, 114.9, 114.6, 111.4, 111.1, 110.7, 110.3, 110.2, 57.3, 50.3, 47.7, 32.3, 27.4; C19H16FN3O3 (353.34): calcd C 64.58, H 4.56, N 11.89%; found C 64.48, H 4.50, N 11.80%.
4g. White amorphous solid (yield 82%); mp: 298–300 °C. IR (KBr) 3352, 3278, 2190, 1720, 1652, 1603, 1469 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.62–7.59 (m, 2H), 7.54–7.49 (m, 1H), 7.38 (m, 4H), 7.23–7.16 (m, 2H), 7.11–6.98 (m, 1H), 6.67 (d, J = 7.5 Hz, 1H), 2.56 (s, 2H), 2.20 (d, J = 11.4 Hz, 2H), 1.06 (s, 3H), 1.04 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 195.6, 176.5, 164.8, 159.2, 143.6, 135.1, 133.6, 130.0, 128.8, 128.5, 127.0, 123.8, 123.4, 117.6, 111.2, 108.9, 57.5, 50.2, 47.0, 32.4, 28.0, 27.4; C25H21N3O3 (411.45): calcd C 72.98, H 5.14, N 10.21%; found C 72.89, H 5.08, N 10.13%.
4h. White amorphous solid (yield 90%); mp: 258–260 °C. IR (KBr) 3360, 3265, 2185, 1718, 1670, 1605, 1472 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.29–7.24 (m, 3H), 7.07–6.93 (m, 3H), 3.14 (s, 3H), 2.58 (s, 2H), 2.12 (d, J = 7.2 Hz, 2H), 1.04 (s, 3H), 1.00 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 195.2, 176.9, 164.6, 159.2, 143.9, 133.9, 128.7, 123.1, 122.8, 117.5, 111.1, 108.5, 57.6, 50.3, 46.8, 32.3, 27.9, 27.4, 26.7; C20H19N3O3 (349.38): calcd C 68.75, H 5.48, N 12.03%; found C 68.67, H 5.38, N 11.95%.
4i. Yellowish white amorphous solid (yield 92%); mp: 232–236 °C. IR (KBr) 3356, 3260, 3156, 2190, 1740, 1697, 1578, 1465 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.31 (s, 2H, NH2), 7.22 (t, J = 7.5 Hz, 1H), 7.07 (d, J = 6.9 Hz, 1H), 6.98 (m, 1H), 6.88 (d, J = 7.8 Hz, 1H), 5.87–5.80 (m, 1H), 5.44 (d, J = 17.4 Hz, 1H), 5.16 (d, J = 10.5 Hz, 1H), 4.32–4.24 (m, 2H), 2.59 (s, 2H), 2.14 (d, J = 9.9 Hz, 2H), 1.04 (s, 3H), 1.01 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 195.2, 176.6, 164.7, 159.2, 143.0, 133.9, 132.1, 128.6, 123.2, 122.7, 117.6, 117.0, 111.0, 109.2, 57.6, 50.3, 46.9, 42.3, 32.3, 27.9, 27.4; C22H21N3O3 (375.42): calcd C 70.38, H 5.64, N 11.19%; found C 70.27, H 5.61, N 11.10%.
4j. Yellowish white amorphous solid (yield 87%); mp: 270–272 °C. IR (KBr) 3364, 3256, 3160, 2187, 1715, 1695, 1480 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.55–7.53 (m, 2H), 7.41–7.31 (m, 5H), 7.18–7.13 (m, 2H), 7.04–7.01 (m, 1H), 6.74 (d, J = 7.8 Hz, 1H), 4.96 (m, 2H), 2.65 (s, 2H), 2.25–2.20 (m, 2H), 0.96 (s, 6H); 13C NMR (75 MHz, DMSO-d6) δC 195.4, 177.1, 164.9, 159.4, 143.0, 136.6, 134.0, 128.8, 128.7, 127.5, 123.3, 123.0, 117.8, 111.1, 109.3, 57.7, 50.3, 47.0, 43.7, 32.4, 28.0, 27.4; C26H23N3O3 (425.47): calcd C 73.39, H 5.45, N 9.88%; found C 73.28, H 5.41, N 9.79%.
4k. White amorphous solid (yield 88%); mp: 280–282 °C. IR (KBr) 3360, 3250, 3172, 2192, 1720, 1690, 1475 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.15–7.08 (m, 3H), 6.95–6.81 (m, 3H), 3.59–3.58 (m, 2H), 2.37 (s, 2H), 2.09–1.91 (m, 2H), 1.04 (t, J = 6.9 Hz, 3H), 0.92 (s, 3H), 0.90 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 194.9, 176.1, 164.3, 158.9, 142.5, 133.9, 128.4, 123.0, 122.3, 117.1, 110.8, 108.3, 57.4, 50.0, 46.4, 34.4, 32.0, 27.7, 27.1, 12.1; C21H21N3O3 (363.40): calcd C 69.41, H 5.82, N 11.56%; found C 69.38, H 5.73, N 11.48%.
4l. Yellowish white amorphous solid (yield 91%); mp: 244–246 °C. IR (KBr) 3317, 3237, 3209, 3155, 2949, 2189, 1724, 1661, 1599 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.52–7.48 (m, 4H), 7.36 (s, 2H, NH2), 7.18–7.09 (m, 2H), 7.00–6.95 (m, 1H), 6.74 (d, J = 7.50 Hz, 1H), 4.90 (s, 2H), 2.62 (s, 2H), 2.25–2.10 (m, 2H), 1.05 (s, 3H), 1.02 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 195.7, 177.4, 165.2, 159.6, 143.0, 136.4, 134.2, 131.9, 130.2, 129.0, 123.7, 123.3, 121.0, 118.0, 111.3, 109.5, 57.9, 50.6, 47.2, 43.4, 32.6, 28.3, 27.7; C26H22BrN3O3 (504.37): calcd C 61.91, H 4.40, N 8.33%; found C 61.82, H 4.37, N 8.25%.
4m. White amorphous solid (yield 86%); mp: 184–186 °C. IR (KBr) 3381, 3281, 3137, 2914, 2189, 1706, 1671, 1591, 1465 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.14–7.08 (m, 3H), 6.93–6.84 (m, 3H), 3.54–3.49 (m, 2H), 3.23 (bs, 4H), 2.38 (s, 2H), 2.00 (d, J = 11.4 Hz, 2H), 1.48–1.46 (m, 2H), 1.12–1.06 (bs, 14H), 0.91 (s, 3H), 0.88 (s, 3H), 0.73 (t, 3H); 13C NMR (75 MHz, DMSO-d6) δC 195.1, 176.7, 164.6, 159.3, 143.5, 134.1, 128.7, 123.3, 122.5, 117.5, 111.2, 107.7, 57.8, 50.4, 46.8, 32.3, 31.7, 29.4, 29.3, 29.1, 28.0, 27.5, 27.3, 26.7, 22.5, 14.4; C31H41N3O3 (503.67): calcd C 73.92, H 8.20, N 8.34%; found C 73.86, H 8.15, N 8.28%.
4n. Yellowish white amorphous solid (yield 84%); mp: 228–230 °C. IR (KBr) 3379, 3291, 3155, 2933, 2189, 1706, 1661, 1599, 1482 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.32–7.26 (m, 3H), 7.09–7.00 (m, 3H), 4.59–4.43 (m, 2H), 3.25 (s, 1H), 2.58 (s, 2H), 2.19–2.06 (m, 2H), 1.03 (s, 3H), 1.00 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 195.2, 176.1, 164.7, 159.3, 142.2, 133.8, 128.7, 123.4, 123.2, 117.3, 111.0, 109.3, 78.1, 74.8, 57.3, 50.3, 46.8, 32.4, 29.6, 28.0, 27.5; C22H19N3O3 (373.40): calcd C 70.76, H 5.13, N 11.25%; found C 70.69, H 5.01, N 11.18%.
4o. White amorphous solid (yield 92%); mp: 252–254 °C. IR (KBr) 3390, 3272, 2923, 2189, 2126, 1716, 1692, 1591, 1478 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.21–7.11 (m, 3H), 7.05–6.92 (m, 3H), 4.49–4.34 (m, 2H), 3.25 (s, 1H), 2.58 (bs, 2H), 2.13 (bs, 2H), 1.84 (bs, 2H); 13C NMR (75 MHz, DMSO-d6) δC 194.9, 175.9, 166.2, 158.8, 141.8, 133.6, 128.3, 123.2, 122.8, 116.9, 111.7, 108.8, 77.8, 74.4, 57.0, 46.5, 36.3, 29.2, 26.8, 19.8; C20H15N3O3 (345.35): calcd C 69.56, H 4.38, N 12.17%; found C 69.48, H 4.31, N 12.10%.
4p. Yellowish white amorphous solid (yield 88%); mp: 274–276 °C. IR (KBr) 3424, 3281, 3165, 2958, 2189, 1716, 1581, 1474 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.33 (s, 2H, NH2), 7.24 (bs, 1H), 7.21 (s, 1H), 6.85 (d, J = 8.1 Hz, 1H), 5.82–5.72 (m, 1H), 5.38 (d, J = 17.1 Hz, 1H), 5.11 (d, J = 10.5 Hz, 1H), 4.32–4.26 (m, 2H), 2.64–2.60 (bs, 2H), 2.22–2.18 (bs, 2H), 1.90 (bs, 2H); 13C NMR (75 MHz, DMSO-d6) δC 195.3, 176.2, 166.8, 158.9, 141.5, 135.8, 131.5, 128.1, 126.5, 123.4, 117.2, 116.8, 111.2, 110.3, 56.6, 46.8, 42.1, 36.3, 26.8, 19.7; C20H16ClN3O3 (381.81): calcd C 62.91, H 4.22, N 11.01%; found C 62.85, H 4.15, N 10.92%.
4q. Yellowish white amorphous solid (yield 82%); mp: 258–260 °C. IR (KBr) 3315, 3236, 3150, 2946, 2192, 1724, 1692, 1590, 1465 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.47–7.40 (m, 4H), 7.28 (s, 2H, NH2), 7.12–7.06 (m, 2H), 6.94–6.89 (m, 1H), 6.68 (d, J = 7.5 Hz, 1H), 4.84 (s, 2H), 2.65 (bs, 2H), 2.22–2.20 (bs, 2H), 1.94–1.90 (bs, 2H); 13C NMR (75 MHz, DMSO-d6) δC 195.2, 176.8, 166.5, 158.8, 142.4, 135.8, 133.7, 131.2, 129.6, 128.3, 123.2, 122.7, 120.4, 117.4, 111.7, 108.7, 57.3, 46.7, 42.7, 36.3, 26.8, 19.8; C24H18BrN3O3 (476.32): calcd C 60.52, H 3.81, N 8.82%; found C 60.48, H 3.75, N 8.78%.
4r. White amorphous solid (yield 89%); mp: 294–296 °C. IR (KBr) 3348, 3272, 2195, 1718, 1675, 1605, 1470 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.58–7.54 (m, 2H), 7.46–7.35 (m, 5H), 7.15–7.13 (m, 2H), 7.02–6.97 (m, 1H), 6.62 (d, J = 7.8 Hz, 1H), 2.65 (bs, 2H), 2.24 (bs, 2H), 1.91 (bs, 2H); 13C NMR (75 MHz, DMSO-d6) δC 195.8, 176.7, 166.8, 159.1, 143.7, 135.2, 133.8, 130.1, 128.9, 128.5, 127.1, 124.0, 123.5, 117.7, 112.4, 108.9, 57.7, 47.1, 36.7, 27.1, 20.2; C23H17N3O3 (383.39): calcd C 72.05, H 4.47, N 10.96%; found C 71.89, H 4.39, N 10.88%.
4s. White amorphous solid (yield 90%); mp: 282–284 °C. IR (KBr) 3361, 3289, 3147, 2199, 1716, 1671, 1599, 1474 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.48 (d, J = 6.9 Hz, 2H), 7.41–7.39 (m, 2H), 7.34–7.29 (m, 5H), 6.66 (d, J = 8.1 Hz, 1H), 2.69 (bs, 2H), 2.28 (bs, 2H), 1.99 (bs, 2H); 13C NMR (75 MHz, DMSO-d6) δC 194.2, 175.3, 165.8, 157.7, 140.8, 134.9, 134.6, 132.6, 129.8, 127.2, 126.1, 125.9, 124.9, 116.1, 113.2, 110.0, 109.6, 55.5, 45.7, 42.2, 35.1, 25.7, 18.5; C24H18BrN3O3 (476.32): calcd C 60.52, H 3.81, N 8.82%; found C 60.48, H 3.75, N 8.71%.
Characterization data of 7a–w
7a. White amorphous solid (yield 90%); mp: 278–280 °C. IR (KBr) 3338, 3136, 2182, 1711, 1643, 1584 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.26 (s, 1H), 10.57 (s, 1H), 7.21 (s, 2H, NH2), 7.17 (s, 1H), 7.01–6.92 (m, 2H), 6.86 (d, J = 7.5 Hz, 1H), 1.48 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 178.4, 162.9, 155.6, 141.9, 135.1, 133.1, 129.3, 124.9, 122.9, 119.1, 110.1, 95.8, 55.6, 47.7, 9.3; C15H11N5O2 (293.28): calcd C 61.43, H 3.78, N 23.88%; found C 61.38, H 3.65, N 23.78%.
7b. White amorphous solid (yield 86%); mp: 294–296 °C. IR (KBr) 3386, 3348, 3141, 2182, 1715, 1644, 1582 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.32 (s, 1H), 10.73 (s, 1H), 7.27 (bs, 3H), 7.11 (s, 1H), 6.90 (d, J = 8.1 Hz, 1H), 1.56 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 178.1, 162.9, 155.6, 140.8, 135.2, 129.3, 126.9, 125.0, 119.0, 111.6, 95.1, 55.0, 48.0, 9.4; C15H10ClN5O2 (327.72): calcd C 54.97, H 3.08, N 21.37%; found C 54.88, H 2.97, N 21.28%.
7c. White amorphous solid (yield 87%); mp: 278–280 °C. IR (KBr) 3323, 3148, 2195, 1730, 1637, 1610, 1593 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.31 (s, 1H), 10.62 (s, 1H), 7.27 (s, 2H, NH2), 7.10–7.04 (m, 1H), 7.00–6.97 (m, 1H), 6.91–6.87 (m, 1H), 1.56 (s, 3H); 13C NMR (75 MHz, DMSO-d6, F coupled 13C spectra) δC 178.4, 162.9, 157.4, 155.6, 147.8, 138.2, 135.2, 135.0, 119.0, 116.0, 115.7, 112.8, 112.5, 111.1, 95.3, 55.2, 48.3, 9.4; C15H10FN5O2 (311.27): calcd C 57.88, H 3.24, N 22.50%; found C 57.79, H 3.15, N 22.43%.
7d. Brown amorphous solid (yield 84%); mp: 282–284 °C. IR (KBr) 3348, 3138, 2181, 1712, 1644, 1609, 1582 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.24 (bs, 1H), 10.66 (s, 1H), 7.32 (d, J = 7.5 Hz, 1H), 7.20 (s, 2H, NH2), 7.14 (s, 1H), 6.78 (d, J = 7.8 Hz, 1H), 1.48 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 177.9, 162.9, 155.5, 141.2, 135.5, 135.2, 132.1, 127.7, 119.0, 114.5, 112.1, 95.1, 55.0, 47.9, 9.4; C15H10BrN5O2 (372.17): calcd C 48.41, H 2.71, N 18.82%; found C 48.33, H 2.65, N 18.78%.
7e. White amorphous solid (yield 92%); mp: >300 °C. IR (KBr) 3399, 3346, 3127, 2180, 1713, 1643, 1583 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.33 (s, 1H), 10.73 (s, 1H), 7.59 (d, J = 8.1 Hz, 1H), 7.34 (s, 1H), 7.29 (s, 2H, NH2), 6.77 (d, J = 8.4 Hz, 1H), 1.59 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 177.8, 162.9, 155.6, 141.7, 138.0, 135.8, 135.2, 133.2, 119.0, 112.6, 95.3, 85.7, 55.1, 47.8, 9.5; C15H10IN5O2 (419.17): calcd C 42.98, H 2.40, N 16.71%; found C 42.91, H 2.35, N 16.65%.
7f. White amorphous solid (yield 80%); mp: 228–232 °C. IR (KBr) 3449, 3306, 3189, 2187, 1690, 1646, 1592 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.40 (s, 1H), 7.64–7.59 (m, 2H), 7.52–7.48 (m, 1H), 7.43–7.28 (m, 5H), 7.21–7.12 (m, 2H), 6.80 (d, J = 7.5 Hz, 1H), 1.65 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 176.0, 162.5, 155.3, 142.6, 135.1, 134.2, 131.8, 129.9, 129.2, 128.4, 126.6, 124.9, 123.9, 118.5, 109.1, 95.1, 55.2, 47.3, 9.2; C21H15N5O2 (369.37): calcd C 68.28, H 4.09, N 18.96%; found C 68.19, H 3.95, N 18.88%.
7g. Yellowish white amorphous solid (yield 83%); mp: 240–244 °C. IR (KBr) 3457, 3138, 2195, 1713, 1648, 1326, 1589 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.40 (s, 1H), 11.35 (s, 1H), 8.25–8.22 (m, 1H), 7.92 (s, 1H), 7.41 (s, 2H, NH2), 7.15 (d, J = 8.7 Hz, 1H), 1.59 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 178.8, 163.1, 155.7, 148.2, 143.5, 135.4, 134.2, 126.7, 120.6, 118.8, 110.6, 94.5, 54.4, 47.9, 9.5; C15H10N6O4 (338.27): calcd C 53.26, H 2.98, N 24.84%; found C 53.18, H 2.85, N 24.78%.
7h. Yellowish white amorphous solid (yield 90%); mp: 244–246 °C. IR (KBr) 3392, 3328, 3167, 2194, 1697, 1641, 1589, 1494, 1403 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.32 (s, 1H), 7.35–7.29 (m, 3H), 7.13–7.03 (m 3H), 5.90–5.81 (m, 1H), 5.32–5.18 (m, 2H), 4.44–4.30 (m, 2H), 1.48 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 176.5, 163.0, 155.7, 142.5, 135.2, 132.3, 132.0, 129.4, 124.8, 123.6, 119.0, 117.6, 109.7, 95.6, 55.5, 47.4, 42.3, 9.5; C18H15N5O2 (333.34): calcd C 64.86, H 4.54, N 21.01%; found C 64.78, H 4.45, N 20.88%.
7i. Yellow amorphous solid (yield 84%); mp: 250–254 °C. IR (KBr) 3306, 3177, 2195, 1698, 1639, 1595, 1493 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.28 (bs, 1H), 7.34–7.23 (m, 3H), 7.14–7.02 (m, 3H), 3.67 (bs, 2H), 1.63–1.59 (m, 2H), 1.45 (s, 3H), 0.92 (m, 3H); 13C NMR (75 MHz, DMSO-d6) δC 176.6, 162.8, 155.6, 142.7, 135.0, 132.3, 129.3, 124.6, 123.3, 118.8, 109.1, 95.6, 55.5, 47.2, 41.5, 21.0, 11.4, 9.3; C18H17N5O2 (335.35): calcd C 64.47, H 5.11, N 20.88%; found C 64.38, H 5.05, N 20.72%.
7j. Yellowish white amorphous solid (yield 86%); mp: 262–264 °C. IR (KBr) 3315, 3227, 2182, 1718, 1657, 1595, 1517 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.31 (bs, 1H), 7.36–7.29 (bs, 3H), 7.10 (bs, 3H), 3.21 (s, 3H), 1.46 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 176.7, 163.0, 155.6, 143.4, 135.1, 132.3, 129.5, 124.6, 123.6, 119.0, 109.1, 95.7, 55.2, 47.4, 26.7, 9.3; C16H13N5O2 (307.30): calcd C 62.53, H 4.26, N 22.79%; found C 62.48, H 4.15, N 22.68%.
7k. White amorphous solid (yield 92%); mp: 260–262 °C. IR (KBr) 3385, 3337, 3139, 2189, 1702, 1638, 1589, 1495 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.27 (bs, 1H), 7.34–7.30 (m, 1H), 7.22 (s, 2H, NH2), 7.13 (d, J = 7.8 Hz, 1H), 7.06–7.02 (m, 2H), 3.76–3.73 (m, 2H), 1.46 (s, 3H), 1.16 (t, J = 6.6 Hz, 3H); 13C NMR (75 MHz, DMSO-d6) δC 176.3, 162.9, 155.7, 142.3, 135.2, 132.6, 129.4, 124.8, 123.4, 118.8, 109.1, 95.6, 55.5, 47.3, 34.9, 13.0, 9.4; C17H15N5O2 (321.33): calcd C 63.54, H 4.71, N 21.79%; found C 63.48, H 4.63, N 21.70%.
7l. Yellowish white amorphous solid (yield 88%); mp: 254–258 °C. IR (KBr) 3328, 3163, 2923, 2188, 1715, 1643, 1540, 1470 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.33 (s, 1H), 7.43 (d, J = 6.9 Hz, 2H), 7.36–7.26 (m, 6H), 7.15–7.04 (m, 3H), 5.06–4.91 (m, 2H), 1.38 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 177.1, 163.1, 155.7, 142.4, 136.5, 135.2, 132.3, 129.4, 129.0, 127.9, 124.8, 123.7, 119.1, 109.8, 95.6, 55.4, 47.5, 43.6, 9.3; C22H17N5O2 (383.40): calcd C 68.92, H 4.47, N 18.27%; found C 68.88, H 4.35, N 18.21%.
7m. White amorphous solid (yield 89%); mp: 258–262 °C. IR (KBr) 3397, 3331, 3272, 2914, 2189, 2128, 1716, 1644, 1591, 1482 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.29 (s, 1H), 7.39–7.34 (m, 1H), 7.28 (s, 2H, NH2), 7.18 (d, J = 7.8 Hz, 1H), 7.13–7.09 (m, 2H), 4.68–4.49 (m, 2H), 3.25 (s, 1H), 1.46 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 176.2, 163.1, 155.5, 141.4, 135.4, 132.2, 129.4, 124.8, 124.0, 118.8, 109.8, 95.6, 78.3, 74.8, 54.9, 47.4, 29.5, 9.5; C18H13N5O2 (331.32): calcd C 65.25, H 3.95, N 21.14%; found C 65.18, H 3.88, N 21.08%.
7n. Yellowish brown amorphous solid (yield 94%); mp: 224–226 °C. IR (KBr) 3342, 3130, 2184, 1715, 1646, 1590 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 10.72 (s, 1H), 7.75 (d, J = 7.8 Hz, 2H), 7.56 (s, 2H, NH2), 7.50–7.45 (m, 2H), 7.33–7.22 (m, 2H), 7.14 (d, J = 7.2 Hz, 1H), 7.01–6.96 (m, 1H), 6.91 (d, J = 7.8 Hz, 1H), 1.50 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 177.9, 161.4, 145.3, 144.3, 142.0, 137.6, 132.5, 129.8, 129.6, 126.9, 125.3, 123.0, 120.5, 118.3, 110.2, 96.7, 56.6, 48.2, 12.1; C21H15N5O2 (369.37): calcd C 68.28, H 4.09, N 18.96%; found C 68.19, H 4.01, N 18.88%.
7o. Yellowish brown amorphous solid (yield 83%); mp: 140–142 °C. IR (KBr) 3451, 3299, 3157, 2923, 2197, 2119, 1706, 1653, 1530, 1482 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.80 (d, J = 7.5 Hz, 2H), 7.69 (s, 2H, NH2), 7.55–7.50 (m, 2H), 7.46–7.34 (m, 2H), 7.31–7.24 (m, 2H), 7.16 (t, J = 7.2 Hz, 1H), 4.76–4.57 (m, 2H), 3.32 (s, 1H), 1.51 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 175.6, 161.6, 145.3, 144.4, 141.5, 137.6, 131.7, 129.8, 127.0, 125.2, 124.1, 120.6, 118.0, 110.0, 96.5, 78.1, 75.0, 55.8, 47.9, 29.7, 12.3; C24H17N5O2 (407.42): calcd C 70.75, H 4.21, N 17.19%; found C 70.69, H 4.15, N 17.10%.
7p. White amorphous solid (yield 87%); mp: 158–162 °C. IR (KBr) 3317, 3192, 2914, 2199, 1716, 1661, 1599, 1492 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.31 (s, 1H), 7.51 (d, J = 8.4 Hz, 2H), 7.37–7.26 (m, 5H), 7.09–7.03 (m, 3H), 4.94–4.91 (m, 2H), 1.34 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 177.1, 163.0, 155.7, 142.2, 136.1, 135.2, 132.3, 131.9, 130.3, 129.4, 124.9, 123.8, 121.1, 119.1, 109.7, 95.5, 55.4, 47.4, 43.0, 9.4; C22H16BrN5O2 (462.29): calcd C 57.16, H 3.49, N 15.15%; found C 57.09, H 3.41, N 15.09%.
7q. Yellowish brown amorphous solid (yield 85%); mp: 190–192 °C. IR (KBr) 3299, 3182, 2914, 2189, 1706, 1644, 1618 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.82–7.77 (m, 2H), 7.68 (s, 2H, NH2), 7.56–7.48 (m, 4H), 7.42–7.24 (m, 5H), 7.13–7.08 (m, 2H), 5.02–4.91 (m, 2H), 1.36 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 176.6, 161.3, 145.4, 144.3, 142.3, 137.6, 136.0, 131.9, 131.8, 130.4, 129.9, 127.1, 125.3, 124.0, 121.2, 120.6, 118.4, 109.9, 96.4, 56.3, 47.9, 43.1, 12.2; C28H20BrN5O2 (538.39): calcd C 62.46, H 3.74, N 13.01%; found C 62.38, H 3.65, N 12.88%.
7r. Yellow amorphous solid (yield 78%); mp: 178–180 °C. IR (KBr) 3391, 3324, 3155, 2195, 1692, 1637, 1585, 1494 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.78 (d, J = 7.8 Hz, 2H), 7.70 (s, 2H, NH2), 7.53–7.48 (m, 2H), 7.44–7.32 (m, 3H), 7.10 (d, J = 8.1 Hz, 1H), 5.89–5.80 (m, 1H), 5.33–5.19 (m, 2H), 4.48–4.32 (m, 2H), 1.52 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 175.4, 161.2, 145.2, 143.8, 141.0, 137.3, 133.6, 131.2, 129.5, 129.3, 127.6, 126.7, 125.1, 120.4, 117.9, 117.6, 111.1, 95.5, 55.4, 47.7, 42.2, 12.0; C24H18ClN5O2 (443.88): calcd C 64.97, H 4.09, N 15.78%; found C 64.89, H 4.02, N 15.65%.
7s. White amorphous solid (yield 86%); mp: 272–274 °C. IR (KBr) 3390, 3320, 3158, 2199, 1697, 1643, 1588, 1493 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.37 (s, 1H), 7.42–7.36 (m, 3H), 7.23 (s, 1H), 7.08 (d, J = 8.4 Hz, 1H), 5.87–5.79 (m, 1H), 5.31–5.18 (m, 2H), 4.45–4.35 (m, 2H), 1.54 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 174.7, 161.5, 154.1, 139.7, 133.7, 132.8, 130.1, 127.8, 126.2, 123.4, 117.3, 116.2, 109.7, 93.3, 53.2, 46.1, 40.8, 8.0; C18H14ClN5O2 (367.78): calcd C 58.78, H 3.84, N 19.04%; found C 58.70, H 3.79, N 18.88%.
7t. White amorphous solid (yield 82%); mp: 196–198 °C. IR (KBr) 3350, 3268, 2923, 2187, 2126, 1710, 1653, 1589, 1479 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.72 (d, J = 6.6 Hz, 2H), 7.65 (s, 2H, NH2), 7.58–7.54 (m, 2H), 7.46 (bs, 2H), 7.30 (bs, 1H), 7.15 (d, J = 6.6 Hz, 1H), 4.69–4.52 (m, 2H), 3.28 (s, 1H), 1.48 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 175.1, 161.5, 145.3, 144.0, 140.6, 137.4, 134.1, 132.4, 129.7, 128.1, 126.9, 120.6, 117.8, 115.9, 111.9, 95.7, 77.6, 75.1, 55.2, 47.9, 29.7, 12.2; C24H16BrN5O2 (486.32): calcd C 59.27, H 3.32, N 14.40%; found C 59.18, H 3.25, N 14.38%.
7u. White amorphous solid (yield 88%); mp: 200–204 °C. IR (KBr) 3361, 3299, 3182, 3129, 2968, 2189, 1698, 1644, 1599, 1492 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 12.32 (s, 1H), 7.38–7.33 (m, 1H), 7.28 (s, 2H, NH2), 7.18 (d, J = 7.8 Hz, 1H), 7.12–7.05 (m, 2H), 3.77 (bs, 2H), 3.59–3.56 (m, 2H), 1.91–1.86 (m, 2H), 1.77–1.75 (bs, 2H), 1.48 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 176.6, 162.7, 155.6, 142.5, 136.9, 132.2, 129.3, 124.7, 123.4, 118.7, 109.1, 95.4, 55.4, 47.2, 34.7, 29.7, 26.0, 25.6, 9.3; C19H18BrN5O2 (428.28): calcd C 53.28, H 4.24, N 16.35%; found C 53.20, H 4.15, N 16.28%.
7v. Brownish white amorphous solid (yield 80%); mp: 228–232 °C. IR (KBr) 3451, 3326, 3192, 2189, 1706, 1653, 1572, 1519 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 10.74 (s, 1H), 7.76 (d, J = 9.0 Hz, 2H), 7.66 (d, J = 9.0 Hz, 2H), 7.59 (s, 2H, NH2), 7.29–7.24 (m, 1H), 7.16 (d, J = 6.9 Hz, 1H), 7.03–6.92 (m, 2H) 1.53 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 177.4, 161.0, 145.1, 144.5, 141.6, 136.6, 132.3, 132.0, 129.3, 124.9, 122.7, 121.9, 118.9, 117.9, 109.9, 96.7, 56.3, 47.8, 11.7; C21H14BrN5O2 (448.27): calcd C 56.27, H 3.15, N 15.62%; found C 56.18, H 3.10, N 15.58%.
7w. Brownish white amorphous solid (yield 84%); mp: 198–202 °C. IR (KBr) 3352, 3192, 2914, 2199, 1716, 1653, 1581, 1519, 1474 cm−1; 1H NMR (300 MHz, DMSO-d6) δH 7.79–7.76 (m, 2H), 7.71–7.68 (bs, 4H), 7.45 (d, J = 8.1 Hz, 2H), 7.25 (d, J = 8.1 Hz, 1H), 3.26 (s, 3H), 1.51 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δC 174.9, 160.6, 144.6, 143.6, 141.3, 135.9, 132.9, 131.7, 128.8, 127.0, 124.4, 121.5, 118.5, 117.1, 109.9, 95.3, 54.7, 47.1, 26.1, 11.2; C22H15BrClN5O2 (496.74): calcd C 53.19, H 3.04, N 14.10%; found C 53.06, H 2.95, N 14.08%.
Acknowledgements
C. B. and A. K. thank UGC, New Delhi, India for offering them Junior Research Fellowship (JRF) and Senior Research Fellowship (SRF) respectively. The financial assistance of CSIR, New Delhi is gratefully acknowledged [Major Research Project, No. 02(0007)/11/EMR-II]. Crystallography was performed at the DST-FIST, India-funded Single Crystal Diffractometer Facility at the Department of Chemistry, University of Calcutta. We also acknowledge Center for Research in Nanoscience and Nanotechnology (CRNN), University of Calcutta for instrumental facilities.
References
-
(a) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS PubMed;
(b) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS PubMed.
-
(a) J. M. Yan, X. B. Zhang, S. Han, H. Shioyama and Q. Xu, Angew. Chem., Int. Ed., 2008, 47, 2287 CrossRef CAS PubMed;
(b) B. C. Ranu, R. Dey, T. Chatterjee and S. Ahammed, ChemSusChem, 2012, 5, 22 CrossRef CAS PubMed;
(c) Z. N. Siddiqui, N. Ahmed, F. Farooq and K. Khan, Tetrahedron Lett., 2013, 54, 3599 CrossRef CAS PubMed.
-
(a) D. Astruc, Inorg. Chem., 2007, 46, 1884 CrossRef CAS PubMed;
(b) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS PubMed;
(c) V. Polshettiwar, B. Baruwati and R. S. Varma, Green Chem., 2009, 11, 127 RSC;
(d) M. T. Reetz and E. Westermann, Angew. Chem., Int. Ed., 2000, 39, 165 CrossRef CAS;
(e) T. Zeng, W. W. Chen, C. M. Cirtiu, A. Moores, G. Song and C. J. Li, Green Chem., 2010, 12, 570 RSC.
- Y. Zhao, W. Li, M. Zhang and K. Tao, Catal. Commun., 2002, 3, 239 CrossRef CAS.
-
(a) A. Kundu, S. Pathak and A. Pramanik, Asian J. Org. Chem., 2013, 2, 869 CrossRef CAS PubMed;
(b) K. Debnath and A. Pramanik, Tetrahedron Lett., 2015, 56, 1654 CrossRef CAS PubMed;
(c) K. Debnath, K. Singha and A. Pramanik, RSC Adv., 2015, 5, 31866 RSC.
-
(a) S. Pathak, K. Debnath and A. Pramanik, Beilstein J. Org. Chem., 2013, 9, 2344 CrossRef PubMed;
(b) K. Debnath, S. Pathak and A. Pramanik, Tetrahedron Lett., 2014, 55, 1743 CrossRef CAS PubMed;
(c) A. Kundu and A. Pramanik, Mol. Diversity, 2015, 19, 459 CrossRef CAS PubMed;
(d) K. Debnath, S. Pathak and A. Pramanik, Tetrahedron Lett., 2013, 54, 896 CrossRef CAS PubMed;
(e) S. Pathak, K. Debnath, M. R. Mollick Md and A. Pramanik, RSC Adv., 2014, 4, 23779 RSC.
-
(a) D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed;
(b) V. Sharma, P. Kumar and D. Pathak, J. Heterocycl. Chem., 2010, 47, 491 CAS;
(c) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed.
-
(a) M. M. Khafagy, A. H. El-Wahas, F. A. Eid and A. M. El-Agrody, Farmaco, 2002, 57, 715 CrossRef CAS;
(b) P. R. Sebahar and R. M. Williams, J. Am. Chem. Soc., 2000, 122, 5666 CrossRef CAS.
- T. H. Kang, K. Matsumoto, Y. Murakami, H. Takayama, M. Kitajima, N. Aimi and H. Watanabe, Eur. J. Pharmacol., 2002, 444, 39 CrossRef CAS.
-
(a) P. E. Maligres, I. Houpis, K. Rossen, A. Molina, J. Sager, V. Upadhyay, K. M. Wells, R. A. Reamer, J. E. Lynch, D. Askin, R. P. Volante and P. J. Reider, Tetrahedron, 1997, 53, 10983 CrossRef CAS;
(b) B. L. Palucki, S. D. Feighner, S. S. Pong, K. K. McKee, D. L. Hrenuik, C. Tan, A. D. Howard, L. H. Y. Vander Ploeg, A. A. Patchett and R. P. Nargund, Bioorg. Med. Chem. Lett., 2001, 11, 1955 CrossRef CAS.
-
(a) S. P. Satasia, P. N. Kalaria, J. R. Avalani and D. K. Raval, Tetrahedron, 2014, 70, 5763 CrossRef CAS PubMed;
(b) P. Rai, M. Srivastava, J. Singh and J. Singh, New J. Chem., 2014, 38, 3181 RSC;
(c) B. M. Rao, G. N. Reddy, T. V. Reddy, B. L. A. P. Devi, R. B. N. Prasad, J. S. Yadav and B. V. S. Reddy, Tetrahedron Lett., 2013, 54, 2466 CrossRef PubMed;
(d) X. Liu, X. Xu, X. Wang, W. Yang, Q. Qian, M. Zhang, L. Song, H. Deng and M. Shao, Tetrahedron Lett., 2013, 54, 4451 CrossRef CAS PubMed;
(e) P. Saluja, K. Aggarwal and J. M. Khurana, Synth. Commun., 2013, 43, 3239 CrossRef CAS PubMed;
(f) R. Baharfar and R. Azimi, Synth. Commun., 2014, 44, 89 CrossRef CAS PubMed;
(g) A. Dandia, V. Parewa, A. K. Jain and K. S. Rathore, Green Chem., 2011, 13, 2135 RSC;
(h) S. Riyaz, A. Naidu and P. K. Dubey, Lett. Org. Chem., 2012, 9, 101 CrossRef CAS;
(i) D. M. Pore, P. G. Hegade, D. S. Gaikwad, P. B. Patil and J. D. Patil, Lett. Org. Chem., 2014, 11, 131 CrossRef CAS;
(j) G. Shanthi, G. Subbulakshmi and P. T. Perumal, Tetrahedron, 2007, 63, 2057 CrossRef CAS PubMed;
(k) C. Han, W. Meng, H. Liu, Y. Liu and J. Tao, Tetrahedron, 2014, 70, 8768 CrossRef CAS PubMed.
- R. Malakooti, H. Mahmoudi, R. Hosseinabadi, S. Petrov and A. Migliori, RSC Adv., 2013, 3, 22353 RSC.
- A. Saha, S. Payra and S. Banerjee, Green Chem., 2015, 17, 2859 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1409892 and 1411963. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra16259a |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent.
1) as the eluent.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent.
1) as the eluent.






