Structural, vibrational study and UV photoluminescence properties of the system Bi(2−x)Lu(x)WO6 (0.1 ≤ x ≤ 1)
H. Ait ahsaine*a,
M. Ezahria,
A. Benlhachemia,
B. Bakiza,
S. Villainb,
J.-C. Valmaletteb,
F. Guinnetonb,
M. Arabb and
J.-R. Gavarrib
aLaboratoire Matériaux et Environnement LME, Faculté des Sciences d'Agadir, Université Ibn Zohr, BP 8106, Cité Dakhla, Agadir, Maroc. E-mail: a.hassan@uiz.ac.ma; Tel: +212 676363621
bInstitut Matériaux Microélectronique et Nanosciences de Provence, IM2NP, UMR CNRS 6242, Université de Toulon, BP 20132, 83957, La Garde Cedex, France
First published on 26th October 2015
Abstract
The bismuth lutetium tungstate series Bi(2−x)Lu(x)WO6 with 0.1 ≤ x ≤ 1 were synthesized by solid state reaction of oxide precursors at 1000 °C for 3 h. The as-prepared polycrystalline compounds were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), Raman spectroscopy and photoluminescence (PL) analyses. Biphasic samples were obtained in the composition range of 0.1 ≤ x ≤ 0.3. Solid solutions were obtained in the composition range of 0.4 ≤ x ≤ 1, and their monoclinic crystal structure was refined using the Rietveld method. SEM micrographs showed that solid solutions presented homogeneous morphologies. Attributions of Raman vibrational modes were proposed. A shift in the vibrational wavenumber depending on the lutetium composition was observed. The specific broadening of the spectral bands was interpreted in terms of long range Bi/Lu disorder and local WO6 octahedron distortions in the structure. The PL experiments were performed under UV-laser light irradiation. Each PL band was decomposed into three Gaussian components with energies close to 1.25, 1.80 and 2.1 eV. Their integrated intensities increased with the value of x. The presence of the near infrared band at 1.25 eV is discussed.
I. Introduction
In the general framework of the development of multifunctional materials for various applications, we focus our attention on the correlations between the structure and luminescence properties of tungstate-based materials. In general, it is well established that extrinsic and intrinsic point defects can play a prominent role in photoluminescence properties.Tungstate materials have been extensively studied and investigated because of their potential applications in numerous fields as functional materials in high-performance luminescent materials,1–4 catalysts,5,6 scintillators,7 laser hosts,8 as well as microwave applications9,10 and humidity sensors.11 Metal tungstates with the general formula AWO4 can be divided into two groups depending on the tungsten environment: (i) scheelite-type structures with WO42− tetrahedral groups and (ii) wolframite-type structures with WO66− octahedral groups.12 Depending on the radius of the A2+ cation, CaWO4,13,14 PbWO4 (ref. 15 and 16) and AREWO4 (with A = alkali metal, RE = rare earth element)17–20 crystallize in the scheelite-type structures, whereas CdWO4,21 ZnWO4,22 MgWO4 (ref. 23) and BaWO4 (ref. 24) crystallize in the wolframite-type structures. Due to charge transfer linked to the tetrahedral or octahedral groups, tungstates can present interesting photoluminescence properties. Tungstates with the general formula RE2WO6 (RE = Y, La, Lu, Gd) belong to the second group with distorted WO6 units and have attracted great attention in the field of luminescence.25
Recently, lutetium-based compounds, such as Lu2SiO5,26 Lu3Al5O12,27 LuAlO3,28 LuBO3,29 Lu2O3 (ref. 30 and 31) and undoped and doped Lu2WO6,32–34 have attracted increasing interest for their potential applications due to their excellent luminescence properties.
In our recent study,35 we synthesized the new monoclinic compound bismuth lutetium tungstate, BiLuWO6, with a structure similar to that of the BiREWO6 (RE = rare earth: Gd, Nd, Y) series. Its structure was characterized by disorder due to the Bi/Lu bonding competition. In a previous study,36 we determined the local structure and the electrical properties of layered phase Bi2WO6. In preliminary studies, we observed that for compositions lower than x = 0.1, a solid solution could be obtained with the orthorhombic structure of Bi2WO6. In the intermediate composition range, we observed a biphasic system with two structures similar to those of Bi2WO6 (orthorhombic) and BiLuWO6 (monoclinic). Finally, for compositions with x > 0.35, we observed a unique monoclinic phase, similar to that of BiLuWO6.
In the present study, we investigate the Bi2WO6–BiLuWO6 system represented by the general formula Bi2−xLuxWO6 with 0.1 ≤ x ≤ 1. We use the Rietveld method to refine the crystal structure of solid solutions with compositions of 0.4 ≤ x ≤ 1 and present the first vibrational analyses from Raman spectroscopy. Finally, we perform the first photoluminescence analyses under UV excitation as a function of the value of x for lutetium.
II. Experimental section
II.1. Synthesis of the material
Bismuth lutetium tungstate compounds were prepared using a solid state reaction process. To prepare the Bi(2−x)Lu(x)WO6 compounds, appropriate amounts of lutetium oxide Lu2O3 (Alfa Aesar Aldrich >99%), bismuth oxide Bi2O3 (Fluka Chemika >99%) and tungsten oxide WO3 (Fluka Chemika >99%) were mixed and milled in an agate mortar, as reported in our earlier paper.35 The mixture was then heated at 1000 °C for 15 hours.II.2. X-ray diffraction
The X-ray diffraction (XRD) patterns were collected using an EMPYREAN PANALYTICAL diffractometer operating at 45 kV/35 mA, using CuKα radiation with a Ni filter in continuous mode with a step size of 0.013°. Data suitable for Rietveld refinement were collected over the 2θ range of 5–90°.II.3. Microstructural characterization
Scanning electron microscopy (SEM) analysis was used to observe the morphology and the local composition of the polycrystalline material. Determination of the chemical compositions was performed using Energy Dispersive Spectroscopy (EDS) in surface scanning mode. Preliminary images were obtained with a SUPRA 40 VP COLONNE GEMINI ZEISS instrument using a maximum voltage of 20 kV.II.4. Raman spectroscopy
Raman spectra (RS) were obtained on a VERTEX 70 Raman spectrometer using a power of 30 mW and the wavelength of Ar green laser λ = 514.5 nm. The frequency bands ν ranged from 50 to 1100 cm−1.II.5. UV photoluminescence
The equipment used to perform the photoluminescence (PL) measurements under UV excitation was a Horiba Jobin-Yvon HR800 LabRam spectrometer. The entrance slit, positioned behind the filter, is a diaphragm whose diameter can range from 50 to 500 μm. The irradiated zone was limited to 1 μm in diameter for all samples. The polycrystalline samples were in the form of compacted pellets obtained under a fixed pressure of 5 kbar. The spherical mirror, characterized by an 800 mm focal length, allows reflecting the scattered radiation from the input to the dispersive grating to obtain a spectra slot. The 364.5 nm (3.40 eV) line of an Ar-ion laser was used as the excitation source. The power applied to the samples was fixed to 0.005 mW with the acquisition time set to 100 ms.III. Results and discussion
III.1. X-ray diffraction analyses
Fig. 1 illustrates the XRD patterns of the synthesized compounds with compositions from x = 0.1 to x = 1. A biphasic system is observed in the composition range of 0.1 ≤ x ≤ 0.3. Characteristic peaks of orthorhombic bismuth tungstate Bi2WO6 were detected in addition to the peaks of the monoclinic BiREWO6 (RE = Lu) phase. It should be recalled that all the as-prepared ceramics were thermally treated at 1000 °C and were highly crystallized. The substituted compounds ranging from 0.4 ≤ x ≤ 1 present a monoclinic structure and constitute a solid solution. This structural modification (orthorhombic/monoclinic) can be explained from the two electronic configurations of Bi3+ and Lu3+; in the case of the Bi3+ cations (electron configuration 6s2 6p0), the lone pair (6s2) could play a spatial role in the structure organization, which is not the case for the Lu3+ cations. In addition, the ionic size of the Lu3+ cations, which is smaller than that of the Bi3+ cations, favors the formation of a solid solution having the structure of the limiting phase, BiLuWO6.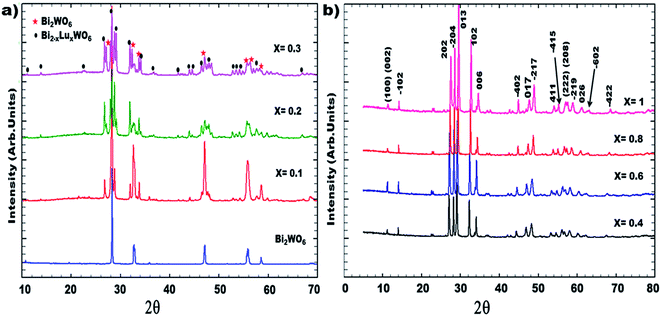 | ||
| Fig. 1 XRD pattern of Bi(2−x)Lu(x)WO6 obtained at room conditions: (a) biphasic system up to x = 0.3; (b) monoclinic solid solution up to x = 1. | ||
The identification of these compounds was firstly obtained from the standard JCPDS files (Joint Committee standards for Powder Diffraction)37 in which the standard phases BiYWO6, BiNdWO6, BiGdWO6 and H–Bi2WO6 were referenced. Fig. 2 shows that the diffraction peaks of the monoclinic solid solution are shifted to higher angles as x increases.
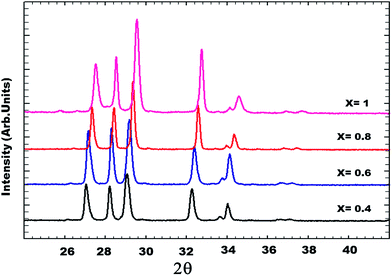 | ||
| Fig. 2 XRD patterns as a function of lutetium composition (x) of the solid solution for 0.4 ≤ x ≤ 1. | ||
The structural parameters of the solid solutions with ‘x’ ranging from 0.4 ≤ x ≤ 1 were refined using FullProf suite software,38 which allows refinement of atomic coordinates, site occupancies and atomic displacement parameters as well as profile parameters (instrument parameters, background parameters, lattice constants and peak shape). The disordered model based on the work of Berdonosov and co-workers39,40 for the series Bi2−xLnxWO6 (Ln = lanthanide) was used in our Rietveld calculations. In this model, Lu atoms are assumed to be distributed on the two bismuth sites of the H–Bi2WO6 monoclinic structure characterized by a centrosymmetric A2/m space group. Such a substitution should involve specific disordered distortions of the octahedral WO6 groups due to alternation of the short Lu–O and long Bi–O bonds.
We refined the atom coordinates of the heavy atoms (Bi/Lu) and W, including their individual Debye factors, while keeping the oxygen coordinates fixed by using the monoclinic A2/m space group. The occupancy factors of all atoms were fixed in agreement with the global composition x. We obtained a significant goodness of fit and the factors Rp, Rexp, Rwp and RB are quite reliable. Table 1 reports the final calculated cell parameters for the solid solution samples. Table 2 summarizes the atom coordinates used for the refinement calculations. In the calculations, each (Bi,Lu) site is assumed to be occupied by Bi and Lu atoms in proportions corresponding to the composition x. The occupancy factors used in the Rietveld procedure are in an arbitrary scale.
| a (Å) | b (Å) | c (Å) | B (°) | Biso (Bi/Lu) | Biso (W) | |
|---|---|---|---|---|---|---|
| Bi1.6Lu0.4WO6 | 8.1683(2) | 3.7797(1) | 16.1564(3) | 102.184(1) | 0.28(3) | 0.33(4) |
| Bi1.4Lu0.6WO6 | 8.1403(2) | 3.7619(3) | 16.0923(3) | 102.209(1) | 0.57(4) | 0.35(2) |
| Bi1.2Lu0.8WO6 | 8.1134(1) | 3.7405(3) | 16.0202(2) | 102.382(1) | 1.11(3) | 0.32(3) |
| BiLuWO6 [ref. 34] | 8.0830(1) | 3.7238(1) | 15.9493(2) | 102.634(2) | 0.72(3) | 0.31(2) |
| Compositions | Atom coordinates (x, y, z) and occupancy factors N (arbitrary scale) | ||||
|---|---|---|---|---|---|
| x | y | z | N(Bi)/N(Lu) N(W) or N(O) | ||
| a Note on the reliability factors: Rp = 100. {∑|yiobs − yicalc|/∑|yiobs|}, Rexp = 100. {[(N − P + C)/∑wi|yiobs|2]1/2}, RB = 100. {∑|Ikobs − Ikcalc|/∑|Ikobs|}, RF = 100. {∑|Fkobs − Fkcalc|/∑|Fkobs|} where N, P and C are the number of observations, parameters and constraints, respectively. | |||||
| x = 1 | Bi/Lu(1) | 0.9224(2) | 0.000 | 0.000(2) | 0.125/0.125 |
| Bi/Lu(2) | 0.3960(2) | 0.000 | 0.3170(2) | 0.125/0.125 | |
| W(1) | 0.2951(3) | 0.4792(3) | 0.4925(3) | 0.250 | |
| Reliability factors | Rp = 7%; Rexp = 3%; RB = 4.99%; RF = 4.74% | ||||
| x = 0.8 | Bi/Lu(1) | 0.9233(3) | 0.000 | 0.3321(2) | 0.150/0.100 |
| Bi/Lu(2) | 0.3943(4) | 0.000 | 0.3176(3) | 0.100/0.150 | |
| W(1) | 0.2960(2) | 0.4450(2) | 0.4970(3) | 0.250 | |
| Reliability factorsa | Rp = 8.35%; Rexp = 7.73%; RB = 6.20%; RF = 4.72% | ||||
| x = 0.6 | Bi/Lu(1) | 0.9266(3) | 0.000 | 0.3327(2) | 0.175/0.075 |
| Bi/Lu(2) | 0.3929(2) | 0.000 | 0.3189(2) | 0.075/0.175 | |
| W(1) | 0.3020(2) | 0.4485(3) | 0.4994(2) | 0.250 | |
| Reliability factorsa | Rp = 9.21%; Rexp = 6.79%; RB = 7.43%; RF = 5.48% | ||||
| x = 0.4 | Bi/Lu(1) | 0.9277(2) | 0.000 | 0.3331(3) | 0.200/0.050 |
| Bi/Lu(2) | 0.3950(3) | 0.000 | 0.3183(2) | 0.05/0.200 | |
| W(1) | 0.2985(3) | 0.4560(3) | 0.4990(4) | 0.250 | |
| Reliability factorsa | Rp = 9%; Rexp = 7.87%; RB = 5.46%; RF = 5.24% | ||||
| Fixed oxygen atoms | O(1) | 0.1188 | 0.000 | 0.2489 | 0.250 |
| O(2) | 0.3640 | 0.500 | 0.2338 | 0.250 | |
| O(3) | 0.3224 | 0.000 | 0.5284 | 0.125 | |
| O(4) | 0.5068 | 0.438 | 0.5745 | 0.250 | |
| O(5) | 0.1709 | 0.414 | 0.5669 | 0.250 | |
| O(6) | 0.1664 | 0.609 | 0.3992 | 0.250 | |
| O(3a) | 0.2978 | 0.000 | 0.4628 | 0.125 | |
Fig. 3 shows the calculated and observed diffraction profiles Yobs and Ycalc of the Bi(1.2)Lu(0.8)WO6 compound. Fig. 4 shows the evolution and the influence of substitution of bismuth by lutetium on the cell parameters. The cell parameters a, b and c varied quasi-linearly with the composition x according to the respective equations:
| a (Å) = −0.1414x + 8.2252 (R2 = 0.999), |
| b (Å) = −0.0945x + 3.8177 (R2 = 0.997), |
| c (Å) = −0.3467x + 16.297 (R2 = 0.999). |
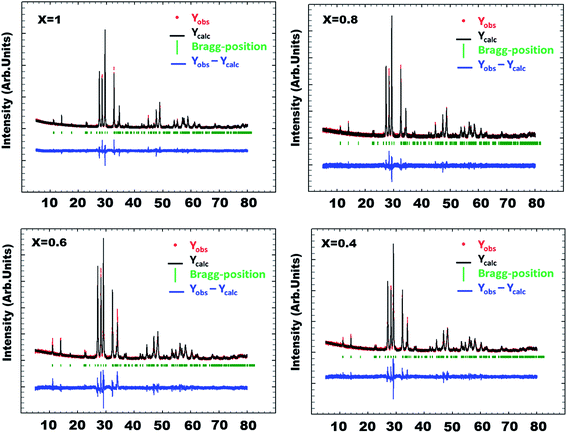 | ||
| Fig. 3 Rietveld refinement of Bi2−xLuxWO6 (x = 0.4, 0.6, 0.8 and 1) using disordered structure and space group A2/m. | ||
The Debye–Waller factor B of the (Bi,Lu) site increases as x increases, with an apparent maximum value at x = 0.8 (Fig. 5). With regard to the standard deviations (close to 10%) of these B values, this increase can be ascribed to the existence of increasing local distortions involving disorder on the (Bi/Lu) site. The disorder can be illustrated by the alternation of different chemical bonds Bi–O and Lu–O, as discussed in ref. 35, 39 and 41.
The monoclinic structure of bismuth lutetium tungstates can be considered as being close to a wolframite-type structure. Fig. 6 shows the alternating (Bi,Lu)2O2 layers and the WO6 edge sharing octahedra. Crystallographic data obtained from our Rietveld calculations showed that the WO6 octahedral complexes have irregular shapes with short, medium and long bonds ranging from 1.65 Å to 2.13 Å.
III.2. Scanning electron microscopy
The SEM micrographs reported in Fig. 7 show the morphology of the synthesized Bi(2−x)Lu(x)WO6:• In the range of 0.1 ≤ x ≤ 0.3, the samples present two types of grain morphologies with small grains similar to those observed in the pure Bi2WO6 samples and micrometric grains similar to those observed in the monoclinic samples (0.4 ≤ x ≤ 1): these two aspects are compatible with the XRD analyses that show the presence of two phases.
• In the solid solution range 0.4 ≤ x ≤ 1, the morphology is quite uniform and consists of rounded grains having dimensions of 1 to 2 μm, as shown in the inset micrographs.
The EDS microanalysis is congruent with the nominal chemical composition of the heavy atoms in Bi(2−x)Lu(x)WO6 and no significant variation in the composition can be observed on the local scale. Table 3 reports the theoretical and the experimental composition of the as-synthesized samples (O atoms were excluded during the EDS analysis). Each EDS experimental fraction is determined with a standard deviation of about 2%.
| Compound | Theoretical compositions in at% | EDS analyses ina at% |
|---|---|---|
| a Note: statistical deviations (in %) of Bi, Lu and W compositions are about: Bi (2–4%)/Lu (4–15%)/W (4%). Determination from series of surface analyses. | ||
| x = 0.1 | Bi (63.33), Lu (3.33), W (33.33) | Bi (64), Lu (3), W (33) |
| x = 0.2 | Bi (60.00), Lu (6.66), W (33.33) | Bi (59), Lu (8), W (33) |
| x = 0.3 | Bi (56.66), Lu (10.00), W (33.33) | Bi (57), Lu (10), W (33) |
| x = 0.4 | Bi (53.33), Lu (13.33), W (33.33) | Bi (53), Lu (14), W (33) |
| x = 0.6 | Bi (46.66), Lu (20.00), W (33.33) | Bi (46), Lu (20), W (34) |
| x = 0.8 | Bi (40.00), Lu (29.33), W (33.33) | Bi (40), Lu (27), W (33) |
| x = 1 | Bi (33.33), Lu (33.33), W (33.33) | Bi (33), Lu (33), W (34) |
III.3. Raman spectroscopy analyses
In the case of the Bi2−xRExWO6 structures, detailed assignments of the vibrational modes were previously proposed by Rocha et al.42 for the bismuth rare earth monoclinic compounds (RE = Y, Nd, Gd). The irreducible representation for these structures is as follows:| Γ = 24Ag + 18Au + 18Bg + 24Bu (44 active Raman modes) |
In general, these Raman modes (i.e., Ag and Bg) can be classified into four categories: (i) symmetric and asymmetric stretching vibrations of the WO6 octahedrons, (ii) bending vibrations of WO6, (iii) stretching and bending vibrations of the (Bi2O2)2+ layers, and (iv) vibrations involving translational motions of Bi3+/Lu3+ and W6+ ions.
Fig. 8 presents the vibrational bands of the compounds belonging to the composition range (0.4 ≤ x ≤ 1): all the compounds exhibit the same vibrational band profiles with a shift to higher wavenumber as the composition of lutetium ‘x’ increases. Table 4 reports the assignments of the Raman bands of the solid solution and the Full Width at Half Maximum (FWHM) of each composition. As the lutetium composition ‘x’ increases, we observe increasing wavenumber and broadening (FWHM) of all vibrational bands. This can be ascribed to the increasing population of Lu–O bonds having stronger force constants with increasing distortions due to the disordered distributions of Lu–O and Bi–O bonds.
| Wavenumber (FWHM) | Assignments [ref. 42] | |||
|---|---|---|---|---|
| x = 0.4 | x = 0.6 | x = 0.8 | x = 1 [ref. 35] | |
| 891 (20) | 897 (25) | 905 (30) | 912 (40) | Asymmetric stretching of WO6 (apical O) |
| 750 (30) | 762 (27) | 770 (20) | 785 (30) | Symmetric stretching of WO6 (apical O) |
| — | 708 (—) | 729 (3) | 731 (5) | |
| 667 (20) | 664 (18) | 665 (20) | 670 (22) | Asymmetric stretching of WO6 (equatorial O) |
| — | — | 647 (—) | 649 (22) | |
| 544 (12) | 550 (10) | 554 (19) | 558 (20) | Bending of WO6 and stretching + bending of (Bi,Lu)O6 polyhedra |
| 500 (22) | 504 (30) | 509 (27) | 511 (20) | |
| 440 (—) | 451 (9) | 459 (10) | 465 (15) | |
| 413 (—) | 417 (10) | 428 (10) | 430 (16) | |
| 347 (18) | 353 (15) | 359 (17) | 364 (15) | Remaining bending of the octahedral oxygen and Bi2O3 layers |
| 286 (21) | 289 (19) | 299 (20) | 303 (12) | |
| 251 (21) | 251 (22) | 249 (20) | 249 (20) | |
| — | — | — | 214 (—) | |
| 189 (11) | 189 (10) | 189 (8) | 191 (10) | |
| 154 (16) | 158 (18) | 160 (17) | 162 (15) | Translation of Bi3+/Lu3+ and W6+ ions lattice modes |
| 77 (21) | 77 (25) | 83 (27) | 89 (35) | |
The vibrational motions of the WO6 octahedra and (Bi/Lu)2O2 layers are directly perturbed by the disorder of the Bi–O and Lu–O bonds induced by the increasing content of lutetium, involving increasing disorder in the oxygen positions of the WO6 octahedra. This should be a major argument in support of the disorder model used in the Rietveld refinements to interpret our XRD data. As already reported for the BiLuWO6 sample, the Raman bands can be highlighted as follows:
• The Raman peaks at 904 and 760 cm−1 can be ascribed to the symmetric and antisymmetric stretching modes of WO6 octahedra, which involve the apical motion of oxygen atoms perpendicular to the layers.
• The peaks in the region of 720–640 cm−1 may be associated with the asymmetric stretching of octahedra, involving equatorial motions of oxygen atoms within layers.43–47
• The bands in the mid-region 370–589 cm−1 represent the bending modes of WO6 and stretching-bending modes of (Bi, Lu)On.
• Some bands are well defined in the spectral range of 180–370 cm−1 and are related to bending modes of the oxygen in the Bi–O polyhedra of the Bi2O2 layers.
• The bands below 180 cm−1 can be ascribed to the translation of Bi3+/Lu3+ and W6+ ions.43,48,49
III.4. Photoluminescence properties
In prior studies, the luminescence of scheelite tungstates was interpreted in terms of electronic charge transfer in the WO42− complex oxyanions or in WO3 defect centers.50 Using a molecular orbital model for the octahedral WO66− oxyanion, von Oosterhout et al.51 showed that the excited state consisted of an electron initially on an oxygen 2p orbital (O2p) and occupying the tungsten 5d orbitals (W5d) with t2g symmetry. Following this model, the emission spectra suggest transitions starting from the double triplet 3T1u levels to the ground 1Ag1 level. Other authors52–55 proposed similar interpretations some of them described the basic emissions through transitions from two triplet states 3T1 and 3T2 to the 1A1 ground state in the case of scheelite structures (charge transfer in WO42− oxyanions) or from two triplet states 3T1u and 3T2u to the 1A1g ground state in the case of wolframite structures (charge transfer in WO66− oxyanions). In the case of octahedral WO66− oxyanions, two additional transitions corresponding to weaker energies were also envisaged. Recently, in our study on Ca1−xCdxWO4 solid solutions,56 we observed photoluminescence bands under UV excitation, localized in the approximate energy range from 2.1 to 2.7 eV.In the case of bismuth-based compounds, the photoluminescence signals under UV excitation were previously ascribed to internal bismuth transitions:57 the authors used theoretical calculations to justify emissions with wavelengths ranging between the UV-visible range 300 and 600 nm and the near infrared (NIR) emissions with wavelengths observed in the approximate range from 800 nm to 1500 nm (and above). NIR emissions were frequently observed in various bismuth-doped materials, including glasses.58,59 These NIR emissions depended on the excitation energies and on the host material itself. The origins of these emissions are unclear to date. According to these authors, these emissions could be generated by bismuth species, such as Bi2+, Bi+ or BiO molecules, as probable defect centers (the list of possible defect centers was not clearly defined by the authors).
The photoluminescence bands obtained under UV excitation (energy of 3.40 eV) are presented in Fig. 9. The emission bands present energies ranging between 1.1 and 2.8 eV. The PL bands were decomposed into three Gaussian components (G1, G2, and G3). The resulting fit is quite satisfactory. The multi-Gaussian decomposition analyses are shown in Fig. 9. Table 5 gives the characteristics of the fitted Gaussian functions: energies of maximum, integrated intensities, and FWHM.
 | ||
| Fig. 9 Luminescence spectra of the polycrystalline Bi(2−x)Lu(x)WO6 phosphors with 0.1 ≤ x ≤ 1, three Gaussian components are shown in each figure. | ||
| Energies of maximum (eV) | Integrated intensity (a.u.) | FWHM (eV) | ||
|---|---|---|---|---|
| X = 0.1 | G1 | 1.279 | 26.8 | 0.08 |
| G2 | 1.791 | 192.3 | 0.22 | |
| G3 | 2.160 | 70.1 | 0.34 | |
| X = 0.2 | G1 | 1.295 | 53.7 | 0.07 |
| G2 | 1.779 | 475.8 | 0.20 | |
| G3 | 2.101 | 161.2 | 0.37 | |
| X = 0.3 | G1 | 1.322 | 69.6 | 0.08 |
| G2 | 1.778 | 770.2 | 0.21 | |
| G3 | 2.022 | 460.0 | 0.29 | |
| X = 0.4 | G1 | 1.321 | 125.4 | 0.09 |
| G2 | 1.781 | 1117.6 | 0.20 | |
| G3 | 1.967 | 546.9 | 0.31 | |
| X = 0.6 | G1 | 1.359 | 127.3 | 0.08 |
| G2 | 1.770 | 1128.7 | 0.21 | |
| G3 | 1.971 | 527.3 | 0.29 | |
| X = 0.8 | G1 | 1.272 | 669.1 | 0.02 |
| G2 | 1.739 | 1250.4 | 0.19 | |
| G3 | 1.941 | 684.3 | 0.29 | |
| X = 1 | G1 | 1.272 | 924.5 | 0.02 |
| G2 | 1.762 | 901.7 | 0.19 | |
| G3 | 1.927 | 627.7 | 0.36 | |
In Fig. 10, each Gaussian function is characterized by its intensity and its energy as a function of the composition x. The energies of the G1 (close to 1.27 eV) and G2 (close to 1.78 eV) components are quasi-independent of the composition x. As x increases, G3 shows a shift to lower energies moving from 2.16 eV to 1.92 eV.
 | ||
| Fig. 10 Integrated intensities and centroid energies of the three Gaussians as a function of lutetium composition. | ||
The G1 component appears to be a narrow band for high lutetium content and is strongly related to the increasing lutetium composition. The origin of the G1 component seems to be unclear. As it has a low energy, Wang et al.60 in their study on ZnWO4 interpreted the broad emission band close to 990 nm (with wavelengths ranging between 850 and 1100 nm) as being related to the presence of defect centers due to oxygen vacancies. In other terms, their broad NIR signal would be related to defect centers on the WO66− groups. However, in our case, the emission band G1, observed at 991 nm or 1.27 eV, is a narrow band. As the intensity of our G1 emission band is strongly conditioned by the lutetium composition, a different interpretation might be proposed. This narrow band could result from a transition due to specific bismuth species acting as defect centers as previously suggested.57,58 In the case of increasing lutetium composition, these bismuth species should appear as more isolated defects giving rise to high sensitization under UV excitation. For a low concentration of lutetium, the low intensity of this G1 component might result from a quenching effect. For high lutetium fractions, i.e., lower fraction of bismuth, this quenching effect might decrease.
The high energetic component G2, which has a maximum at x = 0.8, is attributed to the allowed transition 1T1u → 1A1g of the tungstate groups. Although the G3 component presents the same behavior as the G2 component, the former increases with x till a maximum at x = 0.8, this component could be linked to the 3T1u → 1Ag transitions.
The integrated intensities of the experimental data are shown in Fig. 11. It should be noted that the intensities of all components increase strongly in the composition range of 0.1 ≤ x ≤ 0.4, corresponding to the biphasic system. The total intensity (G1 + G2 + G3) is quasi-constant for compositions of x > 0.4 corresponding to the solid solutions, despite the fact that the intensity of the small G1 component increases slowly as x increases. In the biphasic system, this may be due to the progressive formation of the second (Bi–Lu) phase coexisting with the inactive Bi2WO6 phase, while in the (Bi–Lu) monoclinic solid solution, activation of the luminescence (G1, G2 and G3 components) should occur. As the G2 and G3 intensities remain quasi-constant, this luminescence could be ascribed to charge transfer (or defect centers) linked to the WO6 groups of the monoclinic structure.
VI. Conclusion
In this study, we investigated the complex tungstate system (1 − x)Bi2WO6– xBiLuWO6 or Bi2−xLuxWO6 and observed the existence of a mixed system for x < 0.4 and a solid solution for 0.4 ≤ x ≤ 1. Rietveld refinements of the monoclinic structures of the solid solution system showed that the Lu3+ cations are distributed on the Bi sites. X-ray diffraction analyses coupled with Raman spectroscopy results showed the existence of disordered distributions of Lu and Bi atoms, associated with local distortions.The photoluminescence (PL) under monochromatic UV excitation seems to be strongly related to the formation of the monoclinic structure induced by the substitution of bismuth by lutetium. The PL spectra were decomposed into two types of emissions: the classical emission of tungstate groups WO66− with two components due to charge transfer “W5d → O2p” in the case of octahedral coordination and a specific emission (narrow band at 1.25 eV) strongly related to the presence of lutetium. The exact origin of this NIR emission is not clearly established. If we refer to literature results on tungstates, this NIR emission might be due to defect centers due to oxygen vacancies or defect centers due to bismuth species with different valences or different molecular clustering.
Acknowledgements
A part of this study was financially supported by the CNRS-CNRST project N° 0749/14, Materials and Environment Laboratory (Agadir-Morocco), the Regional Council of Provence-Alpes-Côte d'Azur and the General Council of Var and the Toulon Provence Mediterranean.References
- H. Y. Li, H. K. Yang, B. K. Moon, B. C. Choi, J. H. Jeong, K. Jang, H. S. Lee and S. S. Yi, J. Alloys Compd., 2011, 509, 8788–8793 CrossRef CAS.
- H. Y. Li, H. K. Yang, B. K. Moon, B. C. Choi, J. H. Jeong, K. Jang, H. S. Lee and S. S. Yi, Inorg. Chem., 2011, 50, 12522–12530 CrossRef CAS PubMed.
- V. S. Marques, L. S. Cavalcante, J. C. Sczancoski, A. F. P. Alcantara, M. O. Orlandi, E. Moraes, E. Longo, J. A. Varela, M. S. Li and M. R. M. C. Santos, Cryst. Growth Des., 2010, 10, 4752–4768 CAS.
- W. Zhang, J. Long, A. Fan and J. Li, Mater. Res. Bull., 2012, 47, 3479–3483 CrossRef CAS.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- K. Kamata, T. Kimura, H. Sunaba and N. Mizuno, Catal. Today, 2014, 226, 160–166 CrossRef CAS.
- L. Huo and Y. Chu, Mater. Lett., 2006, 60, 2675–2681 CrossRef CAS.
- H. Zhao, J. Wang, J. Li, H. Zhang, J. Zhang, Z. Ling, H. Xia and R. I. Boughton, Mater. Lett., 2007, 61, 2499–2501 CrossRef CAS.
- J. C. Sczancoski, L. S. Cavalcante, M. R. Joya, J. A. Varela, P. S. Pizani and E. Longo, J. Chem. Eng. Data, 2008, 140, 632–637 CrossRef CAS.
- J. Liu, J. Ma, B. Lin, Y. Ren, X. Jiang, J. Tao and X. Zhu, Ceram. Int., 2008, 34, 1557–1560 CrossRef CAS.
- T. Thongtem, A. Phuruangrat and S. Thongtem, J. Ceram. Process. Res., 2008, 9, 258–261 Search PubMed.
- J. Ungelenk, M. Speldrich, R. Dronskowski and C. Feldmann, Solid State Sci., 2014, 31, 62–69 CrossRef CAS.
- V. B. Mikhailik, H. Kraus, G. Miller, M. S. Mykhaylyk and D. Wahl, J. Appl. Phys., 2005, 97, 083523 CrossRef.
- Y. G. Su, L. P. Li and G. S. Li, Chem. Mater., 2008, 20, 6060–6067 CrossRef CAS.
- J. Yang, C. Lu, H. Su, J. Ma, H. Cheng and L. Qi, Nanotechnology, 2008, 19, 035608 CrossRef PubMed.
- X. L. Hu and Y. J. Zhu, Langmuir, 2004, 20, 1521–1523 CrossRef CAS PubMed.
- F. Lei, B. Yan, H. H. Chen and J. T. Zhao, Inorg. Chem., 2009, 48, 7576–7584 CrossRef CAS PubMed.
- W. Bolanos, J. J. Carvajal, M. C. Pujol, X. Mateos, G. Lifante, M. Aguilo and F. Diaz, Cryst. Growth Des., 2009, 9, 3525–3531 CAS.
- F. Esteban-Betegon, C. Zaldo and C. Cascales, Chem. Mater., 2010, 22, 2315–2324 CrossRef CAS.
- F. Wang, X. P. Fan, D. B. Pi, Z. Y. Wang and M. Q. Wang, J. Solid State Chem., 2005, 178, 825–830 CrossRef CAS.
- Y. Abraham, N. A. W. Holzwarth and R. T. Williams, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 1733–1741 CrossRef CAS.
- H. Ait ahsaine, A. Taoufyq, M. Ezahri, A. Benlhachemi, B. Bakiz, S. Villain, M. Arab, F. Guinneton and J.-R. gavarri, J. Mater. Environ. Sci., 2014, 5, 2449–2454 Search PubMed.
- S. Wannapop, T. Thongtem and S. Thongtem, Appl. Surf. Sci., 2012, 258, 4971–4976 CrossRef CAS.
- W. M. Chance, M. D. Smith and H. C. zur Loye, J. Chem. Crystallogr., 2014, 44, 20–24 CrossRef CAS.
- X.-Y. Chen, Z.-J. Zhang, A. Feng, M. Xu, J.-T. Zhao and F.-F. Xu, Mater. Res. Bull., 2015, 70, 26–31 CrossRef CAS.
- M. Zhuravleva, S. Friedrich and C. L. Melcher, Appl. Phys. Lett., 2012, 101, 101902 CrossRef.
- Y. Zorenko, V. Gorbenko, I. Konstankevych, A. Voloshinovskii, G. Stryganyuk, V. Mikhallin, V. Kolobanov and D. Spassky, J. Lumin., 2005, 114, 85–94 CrossRef CAS.
- D. Singh, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 214115 CrossRef.
- B. I. Zadneprovski, V. V. Sosnovtsev, D. G. Permenov, A. A. Meotishvili and G. I. Voronova, Tech. Phys. Lett., 2009, 35, 815–818 CrossRef CAS.
- N. Kalyvas, P. Liaparinos, C. Michail, S. David, G. Fountos, M. Wójtowicz, E. Zych and I. Kandarakis, Appl. Phys. A, 2011, 106, 131–136 CrossRef.
- H. Ait ahsaine, L. Atourki, M. Ezahri, K. Bouabid, A. Ihlal, S. Villain and A. Benlhachemi, Mater. Lett., 2015, 160, 415–418 CrossRef CAS.
- A. Madej, M. E. Witkowski, A. J. Wojtowicz and E. Zych, J. Lumin., 2015, 160, 50–56 CrossRef CAS.
- X.-Y. Chen, Z.-J. Zhang, L.-L. Zhu, M. Xu, H. Wang, A.-G. Li and J.-T. Zhaoc, Appl. Surf. Sci., 2014, 317, 730–736 CrossRef CAS.
- Z. Zhang, H. Zhang, C. Duan, J. Yuan, X. Wang, D. Xiong, H. Chen and J. Zhao, J. Alloys Compd., 2008, 466, 258 CrossRef CAS.
- H. Ait ahsaine, A. Taoufyq, L. Patout, M. Ezahri, A. Benlhachemi, B. Bakiz, S. Villain, F. Guinneton and J.-R. Gavarri, J. Solid State Chem., 2014, 218, 124–130 CrossRef CAS.
- A. Taoufyq, H. Ait ahsaine, L. Patout, A. Benlhachemi, M. Ezahri, F. Guinneton, A. Lyoussi, G. Nolibe and J.-R. Gavarri, J. Solid State Chem., 2013, 203, 8–18 CrossRef CAS.
- Powder Diffraction File PDF data base sets. JCPDS, International Center for Diffraction Data. Swathmore, P.A, U.S.A. (1994).
- J. Rodriguez-Carvajal, Commission on Powder Diffraction (IUCr) Newsletter, 2001, vol. 26, pp. 12–19 Search PubMed.
- P. S. Berdonosov, D. O. Charkin, K. S. Knight, K. E. Johnston, R. J. Goff, V. A. Dolgikh and P. Lightfoot, J. Solid State Chem., 2006, 179, 3437–3444 CrossRef CAS.
- P. S. Berdonosov, D. O. Charkin, V. A. Dolgikh, S. Yu Stefanovich, R. I. Smith and P. Lightfoot, J. Solid State Chem., 2004, 177, 2632–2634 CrossRef CAS.
- A. Watanabe, Y. Sekikawa and F. Izumi, J. Solid State Chem., 1982, 41, 138 CrossRef CAS.
- G. N. Rocha, L. F. L. Melo, M. C. Castro Jr, A. P. Ayala, A. S. de Menezes and P. B. A. Fechine, J. Phys. Chem., 2010, 71, 579–582 Search PubMed.
- M. Maczka, L. Macalik, K. Hermanowicz, L. Kepinski and P. Tomaszewski, J. Raman Spectrosc., 2010, 41, 1059–1066 CrossRef CAS.
- M. Maczka, L. Macalik and S. Kojima, J. Phys.: Condens. Matter, 2011, 23, 405902 CrossRef CAS PubMed.
- H. C. Gupta, Archana and V. Luthra, J. Mol. Struct., 2011, 1005, 53–58 CrossRef CAS.
- M. Maczka, L. Macalik and J. Hanuza, J. Raman Spectrosc., 2009, 40, 2099–2103 CrossRef.
- M. Maczka, J. Hanuza, W. Paraguassu, A. G. Souza Filho, P. T. C. Freire and J. Mendes Filho, Appl. Phys. Lett., 2008, 92, 112911 CrossRef.
- H. Huang, H. Chen, Y. Xia, X. Tao, Y. Gan, X. Weng and W. Zhang, J. Colloid Interface Sci., 2012, 370, 132–138 CrossRef CAS PubMed.
- L. W. Zhang, Y. J. Wang, H. Y. Cheng, W. Q. Yao and Y. F. Zhu, J. Adv. Mater., 2009, 21, 1286–1290 CrossRef CAS.
- V. Nagirnyi, E. Feldbach, L. Jönsson, M. Kirm, A. Lushchik, C. Lushchik, L. L. Nagornaya, V. D. Ryzhikov, F. Savikhin, G. Svenson and I. A. Tupitsina, Radiat. Meas., 1998, 29, 247–250 CrossRef CAS.
- A. B. J. von Oosterhout, Chem. Phys., 1977, 67, 2412–2418 Search PubMed.
- V. B. Mikhailik, H. Kraus, G. Miller, M. S. Mykhaylyk and D. Wahl, J. Appl. Phys., 2005, 97, 083523 CrossRef.
- V. Nagirnyi, E. Feldbach, L. Jonsson, M. Kirm, A. Kotlov, A. Lushchik, V. A. Nefedov and B. I. Zadneprovski, Nucl. Instrum. Methods Phys. Res., Sect. A, 2002, 486(1), 395–398 CrossRef CAS.
- A. E. Ovechkin, V. D. Ryzhikov, G. Tamulaitis and A. Žukauskas, Phys. Status Solidi A, 1987, 103(1), 285–290 CrossRef CAS.
- R. Grasser, E. Pitt, A. Scharmann and G. Zimmerer, Phys. Status Solidi B, 1975, 69, 359–368 CrossRef CAS.
- A. Taoufyq, F. Guinneton, J.-C. Valmalette, M. Arab, A. Benlhachemi, B. Bakiz, S. Villain, A. Lyoussi, G. Nolibe and J.-R. Gavarri, J. Solid State Chem., 2014, 219, 127–137 CrossRef CAS.
- V. O. Sokolov, V. G. Plotnichenko and E. M. Dian, Opt. Lett., 2013, 33, 1488–1490 CrossRef.
- R. Cao, M. Peng, J. Zheng, J. Qiu and Q. Zhang, J. Opt. Soc. Am., 2012, 16, 18505 Search PubMed.
- B. Xu, D. Tan, S. Zhou, Z. Hong, K. N. Sharafudeen and J. Qiu, J. Opt. Soc. Am., 2012, 20(27), 29105 CAS.
- H. Wang, F. Medina, M. S. Antonious, C. Parkanyi, J. E. Haky, D. M. Baird and Y. D. Zhou, Chem. Phys. Lett., 1993, 205, 497–501 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |






