A computational study to unravel the selectivity in an iron-catalysed [3 + 2] cycloaddition of aziridine and heterocumulenes†
Debashis Adhikari*
Department of Chemistry, Indiana University, Bloomington, Indiana 47405, USA. E-mail: dadhikar@Indiana.edu; Tel: +01-812-855-6779
First published on 29th October 2015
Abstract
The reaction mechanism of cycloaddition between phenyl aziridine and heterocumulene catalysed by iron salts in water has been modeled computationally to trace the origin of the excellent regioselectivity toward 5-substituted product formation. The calculations reveal that the Lewis-acidic iron centre activates and increases the electrophilicity of the heterocumulene upon binding, so that a nucleophilic aziridine-attack can be invoked. The preferential opening of the substituted C2–N bond in the following intermediate, dictated by the stability of an incipient carbocation is the key for such selectivity. Since the aziridine ring-opening step is asynchronous, concerted in nature, the iminoazoselenolidine ring retains the stereopurity at the chiral carbon.
Introduction
Aziridines are very valuable, strained heteroatomic synthons used in preparative organic chemistry based on their ring-opening to a wide variety of synthetic applications.1 The heterocycles such as 2-aryl aziridines are susceptible to nucleophilic ring-opening2 by a metal-catalysed or -mediated process to perform [3 + 2] coupling with heterocumulenes furnishing a wide range of products such as oxazolidines,3 iminothiazolidines,4 iminoazolidinones,5 and iminoimidazolidines.4a In this respect, aziridines behave as masked 1,3-dipoles obtained through C–N bond cleavage. The cycloaddition reactions involving the aziridine ring expansion are very attractive owing to their most atom economical nature where no inorganic waste is generated. Recently, Punniyamurthy and co-workers have significantly expanded the scope of such [3 + 2] coupling reactions by a broad choice of heterocumulenes and synthesizing five-membered heterocycles including iminoazoselenolidine that display important biological6 and medicinal properties (eqn (1)).7
 | (1) |
Their reported reactions are elegant since cheap and environmentally benign iron salts are used as catalysts, and the reactions are performed in water under mild, aerobic conditions. In contrast to the many other reported syntheses involving cycloaddition reactions of similar substrates, this reaction displays complete regio- and stereoselectivity. Despite significant success in two-component cycloadditions involving aziridine, a clear mechanistic picture which can shed light to the origin of regio- and stereoselectivity is lacking. Such an understanding is required to interrogate the role of a substituted aziridine in dictating regioselectivity and controlling stereopurity of the coupled product. In this report, a comprehensive computational study using density functional theory (DFT) has been conducted to unravel the origin of excellent regio- and stereoselectivity of reaction 1.
Computational details
All calculations were carried out using DFT as implemented in the Jaguar 8.0 suite of ab initio quantum chemistry programs.8 Geometry optimizations were performed using the M06 functional9 and the 6-31G** basis set. Iron was represented using the Los Alamos LACVP basis that includes relativistic effective core potentials.10 The energies of the optimized structures were reevaluated by additional single-point energy calculations of each optimized geometry using Dunning's correlation consistent triple-ζ basis set,11 cc-pVTZ(-f) that includes a double set of polarization functions. All stationary points were verified to be minima or transition states by proper vibrational analysis at the double-ζ level.Solvation calculations were carried out with the 6-31G**/LACVP basis at the optimized gas-phase geometry employing a dielectric constants of ε = 80.37 for water. Solvation energies were evaluated using a self-consistent reaction field (SCRF) approach based on accurate numerical solutions of the Poisson–Boltzmann equations.12 For all continuum models, the solvation energies are subjected to empirical parameterization of the atomic radii that are used to generate the solute surface. We employed the standard set13 of optimized radii in Jaguar for H (1.150 Å), C (1.900 Å), O (1.600 Å), Cl (1.974 Å), N (1.600 Å) and Fe (1.456 Å). Although a Born energy reported by COSMO model is a free energy, the entropic contribution accounts for perhaps 2% of the total energy. The solvation enthalpy was therefore taken as the difference between the gas-phase energy and that obtained from COSMO solvation model calculation analytical vibrational frequencies within the harmonic approximation were computed with the 6-31G**/LACVP basis set to confirm proper convergence to well-defined minima or saddle points on the potential energy surface. The free energy of a molecule in solution phase, G(Sol), is computed as follows:
| G(Sol) = G(gas) + Gsolv | (2) |
| G(gas) = H(gas) − TS(gas) | (3) |
| H(gas) = E(SCF) + ZPE | (4) |
| ΔG(Sol) = ∑G(Sol) for products − ∑G(Sol) for reactants | (5) |
To locate transition states, the potential energy surface was first explored approximately using the linear synchronous transit (LST) method,14 followed by a quadratic synchronous transit (QST) search15 that uses the LST transition state as an initial guess. In QST, the initial part of the transition state search is restricted to a circular curve connecting the reactant, initial transition state guess, and the product, followed by a search along the Hessian eigenvectors tangential to this curve. N-Methyl-phenylaziridine has been chosen as a model for the aziridine substrates instead of the N-Pr group used under experimental conditions, to save some computational cost. It is assumed that such a small change will not affect the mechanistic course of the reaction. The iron-complex possesses a sextet spin ground-state and maintains the spin state throughout the entire reaction.
Under the experimental reaction condition, iron-salts are dissolved in water, whereas the organic reactants float on the surface of water.7 The present work focuses on explaining the regioselective outcome of the reaction using standard computational protocols, rather than addressing the dynamics of oil–water interface.
Results and discussion
Given the propensity of FeCl3·6H2O for generating multiple iron-containing species upon dissolution in water, selecting the active form of the catalyst is challenging. At a low concentration of iron salt used under the experimental conditions, FeCl3(H2O)3 should be the predominant species and can be chosen as an active catalyst, as reported from detailed speciation studies by X-ray diffraction.16 Fig. 1 and 2 summarize our computational probation for reaction 1, by extensive computational modelling (DFT), employing M06 density functional.17 The pseudooctahedral FeCl3(H2O)3 loses a labile water ligand to form pentacoordinate iron(III) species which can easily bind to the phenyl isoselenocyanate substrate. Upon binding of this heterocumulene, the electrophilicity of the isoselenocyanate carbon is increased and facilitates the attack of a phenyl aziridine to generate intermediate 4. This intermediate 4 retains the three-membered aziridine ring intact, which is opened by an internal selenide nucleophile preferentially to the phenyl-substituted carbon to form a 5-substituted iminoazoselenolidine. The preferential bond cleavage of the substituted C–N bond in phenyl aziridine by an intramolecular attack of the internal nucleophile in a concerted, asynchronous fashion is responsible for the observed complete regioselectivity (Fig. 1). The novelty of this mechanism lies in two steps: the Lewis-acidic and Lewis-basic interaction between the FeIII-catalyst and phenyl isoselenocyanate, and the intramolecular ring opening of a pendant aziridine by a selenide nucleophile exclusively derived from phenyl isoselenocyanate.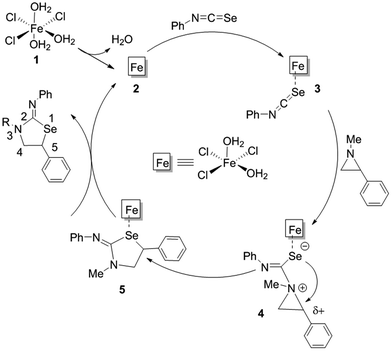 | ||
| Fig. 1 Proposed mechanistic cycle for FeIII-catalyzed coupling of phenyl aziridine and phenyl isoselenocyanate, which has been investigated computationally (DFT, M06 level of theory). | ||
As mentioned earlier, the catalytically competent iron species in water is pseudo-octahedral FeCl3(H2O)6, 1, that forms upon dissolution of iron chloride salt in water. Since both chloride and water are weak field ligands, iron possesses a spin sextet ground-state in this molecule and retains the same spin-state throughout the entire reaction. The catalyst 1 loses one molecule of water to open a vacant coordination site engendering 2 for further substrate binding. The intermediate 2 is slightly higher in energy (1.1 kcal mol−1) than the reference state of the reaction where the catalyst and all substrates are infinitely separated (Fig. 2). The creation of a vacant coordination site is followed by the isoselenocyanate binding to 2 through the selenium. Although this binding is weak and slightly thermodynamically uphill (by 2.9 kcal mol−1), this is sufficient to activate the heterocumulene for further attack by a nucleophile. This activation becomes evident both from elongation of the C–Se bond (by 0.04 Å compared to unbound isoselenocyanate) as well as the increase in electrostatic-potential-fitted (ESP) charge at the carbon by 0.09e. Notably, the binding of phenyl aziridine to the Fe(III) centre is an unproductive mode but can be competitive with the isoselenocyanate binding. Although the phenyl aziridine becomes activated by donation of the nitrogen lone pair to the Lewis-acidic Fe(III) centre, the simple binding is not sufficient to augment the spontaneous ring-opening process (vide infra). The N-methyl-phenyl aziridine then attacks the activated isoselenocyanate carbon to result intermediate 4, where the isoselenocyanate is inserted between the iron and aziridine. This aziridine mediated nucleophilic attack overcomes an energy barrier of 19.3 kcal mol−1. The charge transfer (CT) index for 3-TS, computed from the natural population analysis (NPA) is 0.08e.18 As expected, the successful formation of intermediate 4 by the nucleophilic attack of an aziridine will depend on the nucleophilicity of the nitrogen lone pair of the heterocycle. Indeed, no coupling product was observed experimentally where benzoyl (Bz) and tert-butyloxycarbonyl (Boc) groups were used as N-substituents.7 This can be attributed to heavy delocalization of nitrogen lone pair to the N-substituents, reducing the nucleophilicity of the nitrogen lone pair considerably. The intermediate 4 is significantly zwitterionic in character, as supported from the calculated CT index as 0.51e.18 In the intermediate 4, the Lewis-acidic activation of the phenyl aziridine through an isoselenocyanate moiety becomes very prominent as shown from the following N–C bond length comparison (Fig. 3).
As the difference in elongation of two C–N bonds in 4 suggests, the ring-opening will be preferentially facilitated at the C2 carbon where the presence of a phenyl substitution can stabilize a developing carbocation. Furthermore, the ESP charge separation between C2 and C3 carbons (0.44e) corroborates well with the difference in two C–N bond activation of aziridine in 4. In intermediate 4, the selenide nucleophile19 is appropriately oriented to attack the C2 carbon of the tethered aziridine to form a five-membered iminoazoselenolidine. The large strain energy, 27 kcal mol−1, contained within the three-membered ring20 of aziridine and the required orientation of the nucleophile makes the molecule susceptible for nucleophile-driven ring-opening reaction (Fig. 4). The transition state, 4-TS, leading to the major product formation was located at an energy barrier of 22.3 kcal mol−1.21 Interestingly, the 4-TS is concerted, asynchronous in nature where C⋯N bond cleavage slightly precedes the C⋯Se bond formation. The respective bond distances at the TS are 2.19 and 3.17 Å (Fig. 5, left). The asynchronicity of the TS was further quantified by using eqn (6) where δBi is the bond index for every bond i involved in a chemical reaction. The δBav provides a measure of the degree of advancement of the transition state along the reaction path, and n is the number of bonds that undergo a change during the reaction.22
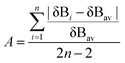 | (6) |
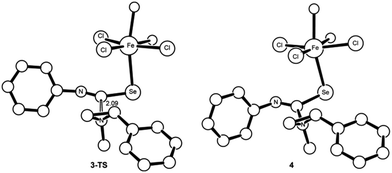 | ||
| Fig. 4 The transition state structure, 3-TS (left) and resulting intermediate 4 (right), as obtained computationally. For clarity all of the hydrogens have been removed. | ||
The asynchronicity index (A) for the major 4-TS is found to be 0.73, which further suggests that the C–N bond cleavage and C–Se bond formation events are not concomitant. The single-bond development index for C–Se bond is calculated to be 0.42, which further ascertains that the TS is late with respect to the new bond formation.23 At the transition state, the loss of energy due to C–N bond cleavage is partly compensated by the electron donation from the selenide nucleophile to the LUMO of the substituted carbon as the delocalization energy from second order perturbation (from NBO analysis) was calculated to be 5.6 kcal mol−1.24 At the TS, the C2 carbon bearing the phenyl substitution is almost planar, as reflected by the angle around it (359.8°) and slight increase in bond order of the C2–Ph bond (from 0.21 to 0.26). It is imperative that the aziridine ring-opening will be preferred at the C2 centre owing to the stability enjoyed by the incipient carbocation. This type of stabilization due to phenyl substitution closely mirrors the allylic25 or the β-silicon stabilization3a of the aziridine carbon during the ring-opening process. Excellent regioselectivity in favour of 5-substituted oxazolidinone formation from the (salen)CrIIICl-catalyzed CO2 and aryl aziridine coupling reaction has also been reported recently, where such a preferential ring-opening of the aryl aziridine seems operative.26 Since the C2–N bond elongation results an incipient carbocation, rather a fully developed one, the stereochemical description of the chiral carbon of aziridine should be retained in the product iminoazoselenolidine. This proposition is completely consistent with the experimental observation: (S)-Ph substituted aziridine reacts with phenyl isoselenocyanate to give optically active iminoazoselenolidine.7
Interestingly, the TS leading to the minor product formation, 4-TS′ (not observed experimentally, reported by NMR detection) is also concerted, asynchronous in character where the developing C⋯Se and cleaving C⋯N bond distances are 2.91 and 2.40 Å respectively.27 The solvation-corrected free-energy difference between these two transition states is 2.4 kcal mol−1, which correctly reproduces the experimental trend. The asynchronicity index for 4-TS′ was calculated to be 0.69 that signifies the minute C–Se bond formation compared to C–N bond cleavage at the TS. Further scrutiny of 4-TS′ structure reveals that the transient carbocation generated from the unsubstituted C3 carbon, being primary in nature, is so electron-deficient that the phenyl group from the adjacent C2 carbon participates via anchimeric assistance28 to confer stability (Fig. 5 right). The participation of the phenyl group to impart stability is evident both from the C2–Ph and C3⋯Ph bond lengths (1.60 and 1.75 Å respectively) as well as a slight change in hybridization (pure sp2 to sp3) for the phenyl carbon to assist better orbital overlap with C3 (see ESI, section S1†). The validity of both the TSs, 4-TS and 4-TS′ were further authenticated by their smooth connection to the intermediate 4 and the final products comprising a 5-membered iminoazoselenolidine ring connected to the Fe(III) centre. Finally, the bound five-membered product is replaced by another molecule of isoselenocyanate and thus the process remains catalytic. Notably, the lack of minor product formation under experimental conditions reiterates the importance of incipient carbocationic stabilization by the presence of phenyl or aryl rings in the aziridine molecule.29 The stability enjoyed by the incipient carbocation from the presence of a –Ph group is critical for the excellent regioselectivity, since alkyl substituted aziridines have been reported to exhibit poor regioselectivity under Lewis-acid mediated- or catalysed conditions.30 Given the discussed stability of the phenyl substituted carbon, it can be hypothesized that the incorporation of more electron donating groups at the para-position of the phenyl ring will increase the incipient carbocationic stability,31 thus increasing the rate of the ring-opening process. This is indeed observed computationally, as the incorporation of a highly electron donating p-OMe group in the same ring decreases the electronic barrier of the step by 6 kcal mol−1 (see ESI, Fig. S1†).
To further ascertain the generality of the proposed mechanism, cycloaddition reactions involving different heterocumulenes were also investigated. Gratifyingly, the heterocumulenes such as phenyl isocyanate and phenyl isothiocyanate exhibit the similar concerted, asynchronous transition states (see ESI, Fig. S2†) leading to major product formation and can be located at reasonable energy barriers (Table 1). The higher reaction energy barrier for these two heterocumulenes compared to that in isoselenocyanates also correlates well with the increasing reaction temperature required for similar [3 + 2] coupling reaction involving them.
| Entry | Heterocumulene | TS-energy (kcal mol−1) |
|---|---|---|
| 1 | Ph-isoselenocyanate | 22.3 |
| 2 | Ph-isocyanate | 28.5 |
| 3 | Ph-isothiocyanate | 31.4 |
Since 4-TS is concerted, asynchronous in character, a true carbocation is never generated during the cycloaddition reaction. As already discussed, stereochemically pure 5-membered iminoazoselenolidine product from stereochemically pure aziridine was indeed observed experimentally. A true carbocation generation should result in the racemization or significant erosion of stereopurity as observed by others in the case of aziridine ring-opening mediated by a strong Lewis acid.3b,32 Our proposed mechanism is reminiscent of Alper's SNi type reaction in a Pd-catalysed ring-opening cycloaddition of aziridines and ketenimines, where a concerted reaction was also invoked as an explanation for the stereochemical retention at the chiral carbon of aziridine4a,33. However, it is noteworthy that the suggested double inversion at the chiral centre for the retention of stereochemistry as proposed by Lee in a chloromethylformate mediated oxazolidinone formation reaction cannot happen in this system.34
Conclusions
The computationally investigated mechanistic course of the cycloaddition reaction between phenyl aziridine and phenyl isoselenocyanate reveals that the activation of aziridine happens through a molecule where the selenocyanate is inserted between catalyst Fe(III) and aziridine. The preferential opening of the aziridine C–N bond at the phenyl-substituted carbon occurs by an internal selenide nucleophile that is responsible for exhibiting excellent regioselectivity. The stereochemical retention of the asymmetric carbon in aziridine to cycloaddition product is a result of the concerted nature of the reaction where a fully developed carbocation is never generated.Acknowledgements
The author gratefully acknowledges generous financial support from Northwestern University and computation time from Indiana University. Thanks are also expressed to Prof. M.-H. Baik (IU) for access to the computational facility.Notes and references
- (a) A. Padwa, Comprehensive Heterocyclic Chemistry III, ed. A. R. Katritzky, C. A. Ramsden and R. J. K. Taylor, Elsevier, Oxford, 2008, vol. 1 Search PubMed; (b) J. B. Sweeney, Chem. Soc. Rev., 2002, 31, 247–258 RSC; (c) A. L. Cardoso and T. M. V. D. Pinho e Melo, Eur. J. Org. Chem., 2012, 2012, 6479–6501 CrossRef CAS.
- (a) S. Stankovic, M. D'Hooghe, S. Catak, H. Eum, M. Waroquier, V. van Speybroeck, N. de Kimpe and H.-J. Ha, Chem. Soc. Rev., 2012, 41, 643–665 RSC; (b) M. K. Ghorai, D. P. Tiwari and N. Jain, J. Org. Chem., 2013, 78, 7121–7130 CrossRef CAS PubMed; (c) X. E. Hu, Tetrahedron, 2004, 60, 2701–2743 CrossRef CAS.
- (a) V. K. Yadav and V. Sriramurthy, J. Am. Chem. Soc., 2005, 127, 16366–16367 CrossRef CAS PubMed; (b) S. Gandhi, A. Bisai, B. A. B. Prasad and V. K. Singh, J. Org. Chem., 2007, 72, 2133–2142 CrossRef CAS PubMed.
- (a) J.-O. Baeg, C. Bensimon and H. Alper, J. Am. Chem. Soc., 1995, 117, 4700–4701 CrossRef CAS; (b) J.-Y. Wu, Z.-B. Luo, L.-X. Dai and X.-L. Hou, J. Org. Chem., 2008, 73, 9137–9139 CrossRef CAS PubMed.
- (a) T. Munegumi, I. Azumaya, T. Kato, H. Masu and S. Saito, Org. Lett., 2006, 8, 379–382 CrossRef CAS PubMed; (b) M. S. Kim, Y.-W. Kim, H. S. Hahm, J. W. Jang, W. K. Lee and H.-J. Ha, Chem. Commun., 2005, 3062–3064 RSC.
- (a) H. Amouri, J. Moussa, A. K. Renfrew, P. J. Dyson, M. N. Rager and L.-M. Chamoreau, Angew. Chem., Int. Ed., 2010, 49, 7530–7533 CrossRef CAS PubMed; (b) V. Jamier, L. A. Ba and C. Jacob, Chem.–Eur. J., 2010, 16, 10920–10928 CrossRef CAS PubMed; (c) C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255–6286 CrossRef CAS PubMed; (d) J. Dubarle-Offner, C. M. Clavel, G. Gontard, P. J. Dyson and H. Amouri, Chem.–Eur. J., 2014, 20, 5795–5801 CrossRef CAS PubMed.
- M. Sengoden and T. Punniyamurthy, Angew. Chem., Int. Ed., 2013, 52, 572–575 CrossRef CAS PubMed.
- Jaguar 8.0, Schrödinger, Inc., Portland, Oregon, 2011 Search PubMed.
- Y. Zhao and D. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS.
- W. R. Wadt and P. J. Hay, J. Chem. Phys., 1985, 82, 284–298 CrossRef CAS.
- T. H. Dunning Jr, J. Chem. Phys., 1989, 90, 1007–1023 CrossRef.
- (a) B. Marten, K. Kim, C. Cortis, R. A. Friesner, R. B. Murphy, M. N. Ringnalda, D. Sitkoff and B. Honig, J. Phys. Chem., 1996, 100, 11775–11788 CrossRef CAS; (b) M. Friedrichs, R. Zhou, S. R. Edinger and R. A. Friesner, J. Phys. Chem. B, 1999, 103, 3057–3061 CrossRef CAS; (c) S. R. Edinger, C. Cortis, P. S. Shenkin and R. A. Friesner, J. Phys. Chem. B, 1997, 101, 1190–1197 CrossRef CAS.
- A. A. Rashin and B. Honig, J. Phys. Chem., 1985, 89, 5588–5593 CrossRef CAS.
- T. A. Halgren and W. N. Lipscomb, Chem. Phys. Lett., 1977, 49, 225–232 CrossRef CAS.
- C. Peng and H. Bernhard Schlegel, Isr. J. Chem., 1993, 33, 449–454 CrossRef CAS.
- M. Magini and T. Radnai, J. Chem. Phys., 1979, 71, 4255–4262 CrossRef CAS.
- (a) Y. Zhao and D. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS; (b) Y. Zhao and D. G. Truhlar, Acc. Chem. Res., 2008, 41, 157–167 CrossRef CAS PubMed.
- L. R. Domingo, RSC Adv., 2014, 4, 32415–32428 RSC.
- J. Moussa, K. M.-C. Wong, X. F. le Goff, M. N. Rager, C. K.-M. Chan, V. W.-W. Yam and H. Amouri, Organometallics, 2013, 32, 4985–4992 CrossRef CAS.
- W. H. Pearson, B. W. Lian and S. C. Bergmeier, Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Elsevier, Oxford, 1996, vol. 1A Search PubMed.
- The M06 functional sometimes slightly underestimates the activation energies. See Tetrahedron, 2012, 68, 8457–8462.
- (a) A. Moyano, M. A. Pericas and E. Valenti, J. Org. Chem., 1989, 54, 573–582 CrossRef CAS; (b) E. Márquez, J. R. Mora, T. Cordova and G. Chuchani, J. Phys. Chem. A, 2009, 113, 2600–2606 CrossRef PubMed; (c) B. Lecea, A. Arrieta, G. Roa, J. M. Ugalde and F. P. Cossio, J. Am. Chem. Soc., 1994, 116, 9613–9619 CrossRef CAS.
- R. Jasiński, Tetrahedron Lett., 2015, 56, 532–535 CrossRef.
- (a) A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS; (b) J. P. Foster and F. Weinhold, J. Am. Chem. Soc., 1980, 102, 7211–7218 CrossRef CAS; (c) A. E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735–746 CrossRef CAS.
- D. C. D. Butler, G. A. Inman and H. Alper, J. Org. Chem., 2000, 65, 5887–5890 CrossRef CAS PubMed.
- (a) A. W. Miller and S. T. Nguyen, Org. Lett., 2004, 6, 2301–2304 CrossRef CAS PubMed; (b) D. Adhikari, A. W. Miller, M.-H. Baik and S. T. Nguyen, Chem. Sci., 2015, 6, 1293–1300 RSC.
- The minor transition state, 4-TS′ starts with an intermediate which is a rotomer of intermediate 4 and isoenergetic to it. In that intermediate the selenium nucleophile is properly oriented toward C3-carbon.
- M. B. Smith and J. March, March's Advanced Organic Chemistry, Wiley-Interscience, New York, NY, 6th edn, 2007 Search PubMed.
- I. Ungureanu, P. Klotz and A. Mann, Angew. Chem., Int. Ed., 2000, 39, 4615–4617 CrossRef CAS.
- (a) R. A. Craig, N. R. O'Connor, A. F. G. Goldberg and B. M. Stoltz, Chem.–Eur. J., 2014, 20, 4806–4813 CrossRef CAS PubMed; (b) M. K. Ghorai, K. Ghosh and K. Das, Tetrahedron Lett., 2006, 47, 5399–5403 CrossRef CAS.
- This is also in accord with Stoltz's observation that N-tosyl-2-mesityl aziridine performs the [3 + 2] cycloaddition with isothiocyanate faster compared to N-tosyl-2-phenyl aziridine. See ref. 30a.
- P. A. Wender and D. Strand, J. Am. Chem. Soc., 2009, 131, 7528–7529 CrossRef CAS PubMed.
- H. Maas, C. Bensimon and H. Alper, J. Org. Chem., 1998, 63, 17–20 CrossRef CAS PubMed.
- T. B. Sim, S. H. Kang, K. S. Lee, W. K. Lee, H. Yun, Y. Dong and H.-J. Ha, J. Org. Chem., 2002, 68, 104–108 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: TS structures, Cartesian coordinates and vibrational frequencies of the structures studied. See DOI: 10.1039/c5ra19407e |
| This journal is © The Royal Society of Chemistry 2015 |



