A novel visible light-driven silver isocyanate photocatalyst: superior stability enhanced by intrinsic resonance effect†
Shuo Fana,
Gang Chen*a,
Chunmei Li a,
Chade Lva,
Zhonghui Hana,
Jiancun Raob,
Yidong Hua and
Congmin Zhanga
a,
Chade Lva,
Zhonghui Hana,
Jiancun Raob,
Yidong Hua and
Congmin Zhanga
aDepartment of Chemistry, Harbin Institute of Technology, 150001, P. R. China. E-mail: gchen@hit.edu.cn; jxsun@hit.edu.cn
bSchool of Materials Science and Engineering, Harbin Institute of Technology, 150001, P. R. China
First published on 21st October 2015
Abstract
Silver isocyanate (AgNCO), a novel visible-light sensitive semiconductor photocatalyst, is prepared based on an intrinsic resonance effect strategy through a simple precipitation process. The as-prepared photocatalyst exhibits photocatalytic degradation ability under visible light. Importantly, it also shows excellent photocatalytic stability, which is a crucial problem to overcome in Ag-based photocatalysts. The existence of an intrinsic resonance effect and its crystal structure may be the main reasons for the superior photocatalytic stability of the AgNCO photocatalyst. The possible transferred and separated behavior of charge carriers and the reason for outstanding photocatalytic stability are illustrated in detail. This study develops a new design idea for exploiting stable Ag-based photocatalysts under visible light irradiation.
Introduction
In recent years, semiconductor photocatalysts, as a type of green solar energy conversion material, have drawn great attention because they can be employed in photodegradating organic contaminants and efficient photocatalytic H2 evolution from water under light irradiation.1–3 Among them, Ag-based photocatalysts are regarded as a new family of promising photocatalytic materials because they exhibit high light utilization rates as well as unique ability to decompose organic pollutants and evolve O2 from water splitting under solar light irradiation.4–7 One of the significant reasons is that the hybridization of the energy band structure is related to participation of the filled d10 electronic configuration of Ag+ ion, which can facilitate the separation and transport of photogenerated electron–hole pairs, thus improving photocatalytic activity.8–11 However, Ag-based photocatalytic materials are sensitive to light, so photocorrosion can occur, which leads to poor photocatalytic stability and decrease of photocatalytic activity.12 To overcome this obstacle, there have been some attempts to inhibit the photocorrosion effect of Ag-based photocatalysts by importing electron acceptors or co-catalysts.13–15 For example, Ag@AgVO3/rGO/PCN reported by Zhang and co-workers displays photodegradation activity and stability for organic dyes.16 A novel series of β-AgAl1−xGaxO2 solid-solution photocatalysts synthesized by Ouyang and Ye shows higher photocatalytic performance than β-AgAlO2 and β-AgGaO2.17 Nevertheless, due to the complexity of the preparation process and inferior stability, it is still a great challenge to develop simple and reliable strategies to produce genuinely stable photocatalysts. To date, novel Ag2Nb4O11 (ref. 18) and Ag2Ta4O11 photocatalysts19 have been reported. Both of these show exceptional photocatalytic stability, but their disadvantage is that they can only respond to the ultraviolet light due to the intrinsic large energy band gap (3.09 and 3.82 eV, respectively). Considering energy utilization and saving, it is particularly important to develop highly efficient visible-light responsive Ag-based semiconductor photocatalysts with better stability.Theoretically, the ligand-to-metal charge transfer (LMCT), which is related to the resonance effect, leads to decrease in the energy of electronic transition, thus expanding the light absorption range.20–22 In addition, internal dipolar fields are believed to aid carrier separation and transport, while the transformation between different intrinsic resonance structures can inhibit the reduction of the Ag+ ion.23 According to reports, the molecular isocyanate NCO− ion commonly used as a reagent in organic and inorganic synthesis has been known for a long time24 and the resonance structures for the isocyanate ligand can be represented in three forms: N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–O− ↔ N−
C–O− ↔ N−![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ↔ N2−–C
O ↔ N2−–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O+.25 Based on the abovementioned reasons, if we adopt the new strategic method in which the molecular isocyanate NCO− ion is combined with the Ag+ ion, novel Ag-based photocatalysts may be equipped with superior photocatalytic stability under visible light irradiation due to intrinsic resonance effect.
O+.25 Based on the abovementioned reasons, if we adopt the new strategic method in which the molecular isocyanate NCO− ion is combined with the Ag+ ion, novel Ag-based photocatalysts may be equipped with superior photocatalytic stability under visible light irradiation due to intrinsic resonance effect.
For the sake of realizing the abovementioned goal, in this study, we prepared a novel Ag-based photocatalyst, AgNCO, through a simple precipitation strategy. The prepared AgNCO sample not only responds to the wide visible-light region but also exhibits superior photocatalytic stability for degradation of dyes. In addition, the positive influences of the intrinsic resonance effect on light absorption, separation efficiency of charge carriers and stability of AgNCO are discussed in detail combined with calculation results based on density functional theory (DFT).
Experimental
Raw materials
The following chemicals were used. Urea (CH4N2O), silver nitrate (AgNO3), titanium oxide (TiO2), Rhodamine B (RhB), methylene blue (MB), benzoquinone (C6H4O2), ammonium oxalate ((NH4)2C2O4·H2O) and dimethyl sulfoxide ((CH3)2SO) were purchased from Aladdin Chemistry Co. Ltd. All reagents were of analytical grade and used as received without further purification. We used distilled water as the solvent.Synthesis of AgNCO sample
AgNCO can be synthesized by a simple precipitation process with AgNO3 solution. In a typical process, 6 g (0.1 mol) urea was first dissolved in 50 mL deionized water to form a clear solution. Then, urea solution was transferred to a three-necked flask, which was placed in an oil bath with a vigorous stirring for 6 hours at 100 °C. 0.85 g (0.005 mol) AgNO3 was dissolved in 50 mL deionized water and placed in the urea solution. After stirring for 10 minutes, the resulting grey suspension was transferred to a beaker and stirred for 20 minutes at room temperature to ensure the reaction was complete. Subsequently, the system was kept static in the dark for 2 hours. The precipitates were washed in turn with secondary distilled water and absolute ethanol to dissolve any unreacted raw materials. Eventually, the precipitated AgNCO products were dried at 60 °C. For comparison, nitrogen-doped TiO2 (N-TiO2) was also synthesized according to a literature method.26Characterization of AgNCO sample
The phase of the prepared AgNCO sample was characterized by powder X-ray diffraction (XRD, RigakuD/max-2000 equipped with a Philips PW3040/60 X-ray diffractometer) at a scanning rate of 5° min−1 in the 2θ range of 10–90° using Cu-Kα radiation. X-ray tube current and voltage were set at 50 mA and 45 kV, respectively. Scanning electron microscopy (SEM) images were acquired using a scanning electron micro-analyzer with an accelerating voltage of 15 kV (FESEM, FEI QUANTA 200F). Energy dispersive X-ray spectrometry (EDS) was performed with a spectroscope attached to the SEM, and used for elemental analysis. Transmission electron microscopy (TEM) of the samples was carried out on an FEI TecnaiG2 S-Twin operating at 300 kV. Further evidence for the composition of the product was obtained from X-ray photoelectron spectroscopy (XPS) using an American electronics physical HI5700ESCA system with an X-ray photoelectron spectroscope using Al-Kα (1486.6 eV) monochromatic X-ray radiation. Fourier transform infrared spectra were obtained utilizing an IR Affinity-1 FT-IR spectrometer. The absorption spectra of the prepared samples were obtained at room temperature using a UV-Vis spectrophotometer (PG, TU-1900) with BaSO4 as the background between 250 nm and 1200 nm at room temperature.Photocatalytic reaction
RhB and MB dyes were used to evaluate the photocatalytic activities of the AgNCO sample. The degradation of RhB and MB dyes were performed in a quartz photochemical reactor under visible light illumination provided by a 300 W Xenon lamp (Trusttech PLS-SXE 300, Beijing) equipped with an ultraviolet cut-off filter and/or band pass filter to provide visible light with λ ≥ 400 nm and/or monochromatic central wavelength visible light with λ of 550 nm and 420 nm (±15 nm), respectively. All experiments were conducted at room temperature in air. In a typical process, RhB solution or MB solution (10 mg L−1, 100 mL) containing 0.1 g AgNCO or 0.1 g N-TiO2 for comparison, was used. After being dispersed in an ultrasonic bath for 5 min, the mixture was stirred for 30 min in the dark to reach adsorption–desorption equilibrium between the catalyst and the solution under continuous magnetic stirring. The suspension was then exposed to visible light irradiation. At every 30 min time interval, 3 mL mixture was collected from the suspension and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min to remove the photocatalysts. The concentrations of RhB or MB were measured from the absorbance at λ = 554 nm or λ = 664 nm with the UV-Vis spectrophotometer (PG, TU-1900).
000 rpm for 5 min to remove the photocatalysts. The concentrations of RhB or MB were measured from the absorbance at λ = 554 nm or λ = 664 nm with the UV-Vis spectrophotometer (PG, TU-1900).
Results and discussion
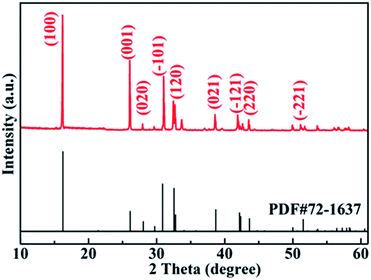
Our design philosophy for the synthesis of AgNCO mainly utilizes the decomposition of urea at a high temperature.27,28 It is reported that if urea solution refluxes for several hours it can generate a large amount of NCO− ions accompanied by a simultaneous rise in pH. The supplementary AgNO3 solution acts not only as a donor of Ag+ ions but also as a pH regulator for the urea solution, therefore avoiding the transformation of NCO− to CO32−. The reaction mechanism of the preparation procedure is shown in Scheme 1.The phase purity and crystallographic structure of the as-prepared AgNCO sample were investigated by XRD. As can be observed in the XRD patterns in Fig. 1, all the diffraction peaks can be indexed satisfactorily to monoclinic AgNCO (JCPDS#72-1637), and no diffraction peaks from impurities are detected. Moreover, the resulting sharp, intense diffraction peaks also indicate the AgNCO sample has high crystallinity. The results demonstrate that an identical crystalline phase AgNCO sample is obtained.
To obtain information on morphology and microstructure of the AgNCO sample, characterization by SEM and TEM was performed. The low magnification SEM image of the AgNCO sample in Fig. 2a shows that the product is composed of rod-like particles. Moreover, the high magnification SEM image in Fig. 2b shows that the surface of the AgNCO sample is smooth. Furthermore, the high angle annular dark field (HAADF) image shown in Fig. 2c confirms the smooth surface of the AgNCO sample, which is consistent with the SEM image. The smooth surface of sample may be beneficial for charge carrier migration.29 The HAADF image of a single AgNCO particle also shows the homogeneity of the elements about the whole particle due to a uniform distribution of light and dark.30,31 Moreover, selected area electron diffraction (SAED) shown in Fig. 2d is performed on the whole particle (shown in Fig. 2f) and the diffraction pattern shows distinctly symmetrical diffraction spots, demonstrating that the AgNCO photocatalyst presents the characteristics of a single crystal.32 In addition, the diffraction spots in the SAED pattern of the sample in Fig. 2d correspond to (−101), (001) and (220) crystal planes in monoclinic AgNCO. The EDS spectrum of Fig. 2e shows that the sample is composed of Ag, N, C and O elements and further proves that pure AgNCO is obtained.
 | ||
| Fig. 2 SEM images (a and b), HAADF image (c), SAED pattern (d), EDS spectrum (e) and TEM image (f) of AgNCO sample. | ||
Furthermore, the elemental composition and chemical state of the AgNCO sample were investigated by XPS. The XPS survey spectra in Fig. S1† show that the sample is composed of Ag, N, C and O elements, which is in accordance with the results from EDS. The high-resolution XPS spectrum of C 1s displayed in Fig. 3a shows two peaks at 284.61 eV and 287.7 eV, which can be correspondingly ascribed to the lattice C 1s state of AgNCO and the carbon of hydrocarbon contaminants, respectively.29 In addition, Fig. 3b shows the high-resolution XPS spectrum of O 1s, with two peaks at 530.8 and 532.1 eV, in line with the lattice O 1s state of AgNCO and adsorbed hydroxyl species, respectively.14 The binding energy peak at 398.6 eV displayed in Fig. 3c is definitely the lattice N 1s state of AgNCO, which can be assigned to sp2-hybridized N bonded to carbon (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–).33 It is found that the peaks of Ag 3d5/2 and Ag 3d3/2 are located at 368.4 eV and 374.4 eV, respectively, in Fig. 3d. Due to the non-broadened and symmetrical peaks, it is believed that silver may only possesses one Ag+ chemical state with no other Ag nanoparticles generated,34 which is in line with the HAADF image.
N–).33 It is found that the peaks of Ag 3d5/2 and Ag 3d3/2 are located at 368.4 eV and 374.4 eV, respectively, in Fig. 3d. Due to the non-broadened and symmetrical peaks, it is believed that silver may only possesses one Ag+ chemical state with no other Ag nanoparticles generated,34 which is in line with the HAADF image.
In addition, the existence and bonding mode of the NCO− anion in AgNCO are detected from the FT-IR spectrum. As shown in Fig. 4, the absorption peaks at 1297 cm−1, 1207 cm−1, and 636 cm−1 are assigned to the pseudosymmetric stretching (C–O) and the bending (N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–O) vibrations for the NCO− ion.35–37 There are two sharp peaks at 2489 cm−1 and 2395 cm−1, which are assigned to the asymmetrical stretching vibration of the cumulative double bond (N
C–O) vibrations for the NCO− ion.35–37 There are two sharp peaks at 2489 cm−1 and 2395 cm−1, which are assigned to the asymmetrical stretching vibration of the cumulative double bond (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).36 It is noted that a strong absorption at 2152 cm−1 matches the characteristic group of frequencies of the antisymmetric stretching of C
O).36 It is noted that a strong absorption at 2152 cm−1 matches the characteristic group of frequencies of the antisymmetric stretching of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N.38–44 However, the sp2-hybridized N bonded to carbon atoms (C
N.38–44 However, the sp2-hybridized N bonded to carbon atoms (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) existing in NCO− is detected by XPS at the same time. The different types of chemical bonds between C and N atoms are perfectly consistent with the resonance formulae of NCO−1, verifying the resonance existence of NCO−1 in AgNCO. The absorption peak at 3448 cm−1 and 3353 cm−1 can be assigned to adsorbed hydroxyl species, which is consistent with the XPS results. The absence of any absorption at 1500 cm−1 in the IR spectrum suggests no presence of CO32− ion in impurity phases,45 which is in accord with the results of XRD. Combining the abovementioned results, it can be concluded that a novel photocatalyst AgNCO has been prepared successfully and the existence of intrinsic resonance effect in AgNCO is confirmed.
N–) existing in NCO− is detected by XPS at the same time. The different types of chemical bonds between C and N atoms are perfectly consistent with the resonance formulae of NCO−1, verifying the resonance existence of NCO−1 in AgNCO. The absorption peak at 3448 cm−1 and 3353 cm−1 can be assigned to adsorbed hydroxyl species, which is consistent with the XPS results. The absence of any absorption at 1500 cm−1 in the IR spectrum suggests no presence of CO32− ion in impurity phases,45 which is in accord with the results of XRD. Combining the abovementioned results, it can be concluded that a novel photocatalyst AgNCO has been prepared successfully and the existence of intrinsic resonance effect in AgNCO is confirmed.
The light absorption capacity of the AgNCO sample is measured by the UV-Vis absorption spectrum. Fig. 5 shows that the absorption edge of AgNCO exists at around 530 nm. The semiconductor band gap of AgNCO is estimated with empirical equation αhν = A(hν − Eg)n, where α, ν, A, Eg, and n are the absorption coefficient, incident light frequency, constant, band gap, and an integer, respectively. The value of n determines the characteristics of the transition in a semiconductor. We define AgNCO as belonging to the indirect bandgap semiconductors and the value of n is calculated as 2, which is also demonstrated by the theoretical calculation result below. Therefore, the bandgap of AgNCO is estimated to be 2.6 eV shown in the inset of Fig. 5.46–47 Moreover, valence band (VB) and conduction band (CB) positions are calculated by means of the empirical formula EVB = X − Ee + 0.5 Eg and ECB = EVB − Eg, where EVB, X, Ee, Eg and ECB are the energy of the VB edge potential, the absolute electronegativity, free electrons on the hydrogen scale (4.5 eV), the band gap energy and the CB edge potential of the semiconductor, respectively. Consequently, ECB and EVB are estimated to be 0.52 eV and 3.12 eV, respectively. It is worth noting that there is a considerable absorption of visible light by the sample in the wavelength range from 550 to 850 nm (shown in Fig. 5), which can be attributed to the ligand-to-metal charge transfer (LMCT) absorptions of AgNCO.20–22 Simultaneously, ligand polarization exists in AgNCO caused by the intrinsic resonance effect from the shift of electrons among N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–O− ↔ N−
C–O− ↔ N−![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ↔ N2−–C
O ↔ N2−–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O+. We surmise that the different intrinsic resonance structures of the NCO− ligand will affect LMCT absorptions in turn due to a change of electron density donated to the metal.48,49 In conclusion, LMCT actually affects the UV-visible absorption and thus AgNCO exhibits a significant enhancement in light absorption intensity in the whole visible light region.
O+. We surmise that the different intrinsic resonance structures of the NCO− ligand will affect LMCT absorptions in turn due to a change of electron density donated to the metal.48,49 In conclusion, LMCT actually affects the UV-visible absorption and thus AgNCO exhibits a significant enhancement in light absorption intensity in the whole visible light region.
 | ||
| Fig. 5 UV-visible spectra and the plots of the (αhν)1/2 versus photon energy (inset) of AgNCO sample. | ||
The photocatalytic activity of the AgNCO sample was investigated by the photocatalytic degradation experiments of the organic dye RhB in aqueous solutions under visible light irradiation. The degradation kinetics curves of RhB solution (Fig. 6a) show that the photolysis is negligible without photocatalyst. Moreover, the concentration of RhB decreases relatively slowly in the dark compared to that under visible light irradiation. Furthermore, AgNCO exhibits improved degradation efficiency compared to that of N-TiO2, and can almost completely remove RhB within 270 min. Moreover, from the UV-visible spectral changes of RhB in Fig. S2,† the apparent decrease in absorption of RhB dye with an accompanying wavelength shift of the band to shorter wavelengths confirms that the dye is de-ethylated in a stepwise manner.50 As the pollutant is limited to the millimolar concentration range, the reaction kinetics of RhB photodegradation by the prepared photocatalysts can be described by pseudo first-order kinetics in terms of the Langmuir–Hinshelwood model51 (eqn (1))
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ct = −kt + ln Ct = −kt + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C0 C0
| (1) |
The purpose of recycling experiments is to detect the stability and re-usability of the samples. Taking the practical application into consideration, the photocorrosion of Ag-based photocatalysts has been highlighted. Therefore, it is particularly important for the Ag-based photocatalyst to have an exceptional photocatalytic stability. Herein, the stability of photocatalysts for the degradation of RhB is chosen. The result is shown in Fig. 6d; there seems to be only a little loss of the photocatalytic activity over the AgNCO sample after 7 cycles in about 30 hours, which verifies that the prepared AgNCO sample has unique photocatalytic stability under visible light. The XRD pattern of the AgNCO sample after 7 cycles is nearly unchanged compared with the fresh sample (Fig. 7) and no Ag phase is detected, which also indicates that AgNCO is a relatively stable Ag-based photocatalyst. Moreover, even when MB dye serves as the target molecule, the removal rate is also higher than that of N-TiO2, as shown in Fig. S3a.† It can almost entirely remove MB within 240 min. The rate constant of the photodegradation of MB over AgNCO is 8.41 × 10−3 min−1, as displayed in Fig. S3c,† which reaches 3.28, 16.8 and 25.4 times that of N-TiO2 (2.56 × 10−3 min−1), that in the dark (5.01 × 10−4 min−1) and that of the blank (3.31 × 10−4 min−1), respectively. Note that there is almost no shift of absorption peak located at 667 nm, as shown in Fig. S3d,† indicating that the benzene/heterocyclic rings of the MB molecule are decomposed into small organic/inorganic molecules or ion products.53 The abovementioned results indicate that the AgNCO photocatalyst exhibits universality for the degradation of various organic dyes, enhanced photocatalytic degradation ability and excellent photocatalytic stability.
The possible photocatalytic reaction mechanism and the carrier separation behaviour promoted by the intrinsic resonance effect over the AgNCO sample are shown in Fig. 8. Considering that the atomic partial charges are not symmetrically distributed in the isocyanate anion,54 intrinsic resonance effect in conjunction with internal dipolar fields is believed to aid carrier separation and transport. Moreover, the band gap of AgNCO is 2.4 eV and thus can be excited under visible light radiation. The charge carrier separation and transfer behaviour suffered from a cycle shown in Fig. 8. First, the electron from the valence-band maximum (VBM) composed of Ag 5d, N 2p and O 2p is excited into the conduction-band minimum (CBM) composed of Ag 5s and N 2s,23 resulting in electron–hole pair formation (Step 1). Then, due to the intrinsic resonance effect of AgNCO and strong electronegativity of O, an internal dipolar field is generated, which can promote the separation of electron and hole. Electron transfers from Ag on the left and one pi bond of O![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C breaks to convert the N2−–C
C breaks to convert the N2−–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O+ group to an N−
O+ group to an N−![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group (Step 2). Subsequently, the electron will react with oxygen to generate superoxide radicals (˙O2−). The ˙O2− active species and ˙OH radicals are responsible for the degradation of RhB and MB, respectively (shown in Fig. S4†). Ultimately, the N−
O group (Step 2). Subsequently, the electron will react with oxygen to generate superoxide radicals (˙O2−). The ˙O2− active species and ˙OH radicals are responsible for the degradation of RhB and MB, respectively (shown in Fig. S4†). Ultimately, the N−![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group reverts to the initial N2−–C
O group reverts to the initial N2−–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O+ state, and the cycle finishes under visible light (Step 3) and then continues. Once electrons and holes have migrated away from their production sites, these abovementioned processes may accelerate electrons and holes to participate in the reaction with oxygen and dyes in time. The transformation between different intrinsic resonance structures reduces the reaction opportunity of electrons and the Ag+ ion. In consequence, the intrinsic resonance effect suppresses the reduction of the Ag+ ion and effectively increases the photocatalytic stability of AgNCO.
O+ state, and the cycle finishes under visible light (Step 3) and then continues. Once electrons and holes have migrated away from their production sites, these abovementioned processes may accelerate electrons and holes to participate in the reaction with oxygen and dyes in time. The transformation between different intrinsic resonance structures reduces the reaction opportunity of electrons and the Ag+ ion. In consequence, the intrinsic resonance effect suppresses the reduction of the Ag+ ion and effectively increases the photocatalytic stability of AgNCO.
 | ||
| Fig. 8 The possible photocatalytic reaction mechanism and the carrier separation behavior over AgNCO sample. | ||
Furthermore, to confirm the position of the electrons and holes of the AgNCO photocatalyst, we calculated the band structure and the density of states (DOS) for Ag, C, O and N near the Fermi level (EF) using first-principles DFT shown in Fig. 9 (see calculation method of theory in ESI†). The corrected band gap dispersion of AgNCO in Fig. 9a proves that AgNCO belongs to an indirect band gap semiconductor in accord with the result shown in the absorption spectrum.55 The calculated partial DOS suggests that the valence band is mainly composed of Ag 4d orbitals hybridized with N 2p, N 2s, O 2s, O 2p, C 2s and C 2p orbitals, and the conduction band primarily consists of Ag 5s, Ag 5p, N 2p, N 2s, C 2p and O 2p orbitals. More specifically, it indicates that the VB of AgNCO is dominated by Ag 4d, N 2p, O 2p and the CB consists of Ag 5s and N 2s, which proves the accuracy of the proposed photocatalytic reaction mechanism mentioned above.56 At the same time, the hybridization of orbitals favors high electron mobility, which may avoid the reduction of Ag+ ions.
The crystal structure of AgNCO is another important factor to enhance the exceptional photocatalytic stability, according to reports.57 As is shown in Fig. 10, the AgNCO compound is composed of zig-zag chains along the Y-axis in which the silver cation is in a two-fold coordination of –(O2C2N2)Ag(N2C2O2)–.25 The NCO−1 acts as a bridge in contact with Ag atoms on both sides of the N, leading to a 120° angle of N–Ag–N formation, which acts in favor of the migration of electrons and holes.16 Moreover, the high electron mobility, the strong electronegativity of the terminal N in the N–Ag–N bond and the transformation between different intrinsic resonance structures may inhibit the non-localized electrons from reacting with Ag+ ions and thus prevent the Ag+ ion from becoming reduced. As mentioned above, this compound contains zig-zag Ag–N–Ag chains with a bond angle of 95.847°, which prevents the overlap of the electron cloud of adjacent atoms of Ag–Ag to protect Ag+ from reducing.24 In addition, the layered structure of AgNCO may facilitate the transport and separation of the carriers.58 All the above features are beneficial to strengthen the photocatalytic stability of AgNCO for the degradation of RhB and MB dyes.
Conclusions
In summary, we have successfully synthesized a novel visible-light sensitive Ag-based photocatalyst, AgNCO, by a simple precipitation reaction. The prepared AgNCO exhibits enhanced photocatalytic activity and excellent photocatalytic stability for the degradation of RhB and MB under visible light irradiation. The superior photocatalytic stability may be attributed to the intrinsic resonance effect and crystal structure of AgNCO. The intrinsic resonance effect can promote the separation and migration of photogenerated electrons and holes, thus improving the photocatalytic activity. This study develops a novel photostabilized Ag-based photocatalyst employing the intrinsic resonance effect under visible light irradiation, which may play a guiding role in the development of Ag-based photocatalysts.Acknowledgements
This study was financially supported by projects of the Natural Science Foundation of China (21271055 and 21471040) and the Fundamental Research Funds for the Central Universities (HIT. IBRSEM. A. 201410). We acknowledge the support by Open Project of State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology (No. QAK201304) and Program for Innovation Research of Science in Harbin Institute of Technology (PIRS of HIT B201412).Notes and references
- S. Q. Liu, Z. R. Tang, Y. G. Sun, J. C. Colmenares and Y. J. Xu, Chem. Soc. Rev., 2015, 44, 5053–5075 RSC.
- K. Maeda, K. Teramura, D. L. Lu, N. Saito, Y. Inoue and K. Domen, Angew. Chem., Int. Ed., 2006, 45, 7806–7809 CrossRef CAS PubMed.
- C. M. Li, G. Chen, J. X. Sun, Y. J. Feng, J. J. Liu and H. J. Dong, Appl. Catal., B, 2015, 163, 415–423 CrossRef CAS.
- J. J. Li, Y. L. Xie, Y. J. Zhong and Y. Hu, J. Mater. Chem. A, 2015, 3, 5474–5481 CAS.
- Y. P. Bi, S. X. Ouyang, N. Umezawa, J. Y. Cao and J. H. Ye, J. Am. Chem. Soc., 2011, 133, 6490–6492 CrossRef CAS PubMed.
- L. Ni, M. Tanabe and H. Irie, Chem. Commun., 2013, 49, 10094–10096 RSC.
- K. Maeda, A. K. Xiong, T. Yoshinaga, T. Ikeda, N. Sakamoto, T. Hisatomi, M. Takashima, D. L. Lu, M. Kanehara, T. Setoyama, T. Teranishi and K. Domen, Angew. Chem., 2010, 122, 4190–4193 CrossRef.
- S. X. Ouyang and J. H. Ye, J. Am. Chem. Soc., 2011, 133, 7757–7763 CrossRef CAS PubMed.
- H. F. Shi, Z. S. Li, J. H. Kou, J. H. Ye and Z. G. Zou, J. Phys. Chem. C, 2011, 115, 145–151 CAS.
- J. Boltersdorf, T. Wong and P. A. Maggard, ACS Catal., 2013, 3, 2943–2953 CrossRef CAS.
- M. Li, J. Zhang, W. Dang, S. K. Cushing, D. Guo, N. Wu and P. Yin, Phys. Chem. Chem. Phys., 2013, 15, 16220–16226 RSC.
- T. Y. Huang, Y. J. Chen, C. Y. Lai and Y. W. Lin, RSC Adv., 2015, 5, 43854–43962 RSC.
- P. H. Wang, Y. X. Tang, Z. L. Dong, Z. Chen and T. Lim, J. Mater. Chem. A, 2013, 1, 4718–4727 CAS.
- N. Zhang, M. Q. Yang, S. Q. Liu, Y. G. Sun and Y. J. Xu, Chem. Rev., 2015, 115, 10307–10377 CrossRef CAS PubMed.
- L. Yuan and Y. J. Xu, Appl. Surf. Sci., 2015, 342, 154–167 CrossRef CAS.
- S. W. Zhang, J. X. Li, X. K. Wang, Y. S. Huang, M. Y. Zeng and J. Z. Xu, J. Mater. Chem. A, 2015, 3, 10119–10126 CAS.
- H. Tong, S. X. Ouyang, Y. P. Bi, N. Umezawa, M. Oshikiri and J. H. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed.
- H. J. Dong, G. Chen, J. X. Sun, Y. J. Feng, C. M. Li and C. D. Lv, Chem. Commun., 2014, 50, 6596–6599 RSC.
- H. J. Dong, J. X. Sun, G. Chen, C. M. Li, Y. D. Hu and C. D. Lv, Phys. Chem. Chem. Phys., 2014, 16, 23915–23921 RSC.
- C. C. Chen, W. H. Ma and J. C. Zhao, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC.
- G. Zhang, G. Kim and W. Y. Choi, Energy Environ. Sci., 2014, 7, 954–966 CAS.
- Y. K. Shin, B. S. Brunschwig, C. Creutz and N. Sutin, J. Am. Chem. Soc., 1995, 117, 8668–8669 CrossRef CAS.
- W. Zhao, Y. F. Liu, J. J. Liu, P. Chen, W. Chen, F. Q. Huang and J. H. Lin, J. Mater. Chem. A, 2013, 1, 7942–7948 CAS.
- J. Nelson and S. M. Nelson, J. Chem. Soc., 1969, 1597–1603 RSC.
- D. J. Williams, S. C. Vogel and L. L. Daemen, Phys. B, 2006, 385, 228–230 CrossRef.
- Z. G. Yi, J. H. Ye, N. Kikugawa, T. Kako, S. X. Ouyang, H. Stuart-Williams, H. Yang, J. Y. Cao, W. J. Luo, Z. S. Li, Y. Liu and R. L. Withers, Nat. Mater., 2010, 9, 559–564 CrossRef CAS PubMed.
- M. Angeles Larrubi, G. Ramis and G. Busc, Appl. Catal., B, 2000, 27, 145–151 CrossRef.
- A. Alahmad, M. Eleoui, A. Falah and I. Alghoraibi, Phys. Rev. Res. Int., 2013, 1, 89–96 Search PubMed.
- H. J. Dong, G. Chen, J. X. Sun, C. M. Li, Y. G. Yu and D. H. Chen, Appl. Catal., B, 2013, 134, 46–54 CrossRef.
- M. Porcu, A. K. Petford-Long and J. M. Sykes, J. Alloys Compd., 2008, 453, 341–346 CrossRef CAS.
- M. K. Devaraju, Q. D. Truong and I. Honma, RSC Adv., 2013, 3, 20633–20638 RSC.
- J. S. Jie, W. J. Zhang, Y. Jiang, X. M. Meng, Y. Q. Li and S. T. Lee, Nano Lett., 2006, 6, 1887–1892 CrossRef CAS PubMed.
- Y. P. Liu, L. Fang, H. D. Lu, Y. W. Li, C. Z. Hu and H. G. Yu, Appl. Catal., B, 2012, 115, 245–252 CrossRef.
- Z. H. Chen, F. Bing, Q. Liu, Z. G. Zhang and X. M. Fang, J. Mater. Chem. A, 2015, 3, 4652–4657 CAS.
- A. Popitsch, E. Nachbaur and H. P. Fritzer, J. Mol. Struct., 1986, 143, 41–46 CrossRef CAS.
- A. Maki and J. C. Decius, J. Chem. Phys., 1958, 28, 1003–1004 CrossRef CAS.
- J. Martínez-Lillo, D. Armentano, G. D. Munno, F. Lloret, M. Julve and J. Faus, Inorg. Chim. Acta, 2006, 359, 4343–4345 CrossRef.
- B. Mavis and M. Akinc, Chem. Mater., 2006, 18, 5317–5325 CrossRef CAS.
- N. J. Nelson, N. E. Kime and D. F. Shriver, J. Am. Chem. Soc., 1969, 91, 5173–5275 CrossRef CAS.
- B. Mavis and M. Akinc, J. Am. Ceram. Soc., 2006, 89, 471–477 CrossRef CAS.
- D. C. Nicola, Effendy, F. Fazaroh, C. Pettinari, B. W. Skelton, N. Somers and A. H. White, Inorg. Chim. Acta, 2005, 358, 720–734 CrossRef.
- P. Rabu, S. Angelov, P. Legoll, M. Belaiche and M. Drillon, Inorg. Chem., 1993, 32, 75–78 CrossRef.
- T. N. Ramesh and P. Vishnu Kamath, J. Power Sources, 2006, 156, 655–661 CrossRef CAS.
- C. L. Schmidt and M. Jansen, J. Mater. Chem., 2010, 20, 110–116 RSC.
- Y. Du and D. O'Hare, Inorg. Chem., 2008, 47, 3234–3242 CrossRef CAS PubMed.
- J. X. Sun, G. Chen, Y. X. Li, R. C. Jin, Q. Wang and J. Pei, Energy Environ. Sci., 2011, 4, 4052–4060 CAS.
- S. Bai, J. Jiang, Q. Zhang and Y. J. Xiong, Chem. Soc. Rev., 2015, 44, 2893–2939 RSC.
- K. M. Milum, Y. N. Kim and B. J. Holliday, Chem. Mater., 2010, 22, 2414–2416 CrossRef CAS.
- C. V. Sastri, M. J. Park, T. Ohta, T. A. Jackson, A. Stubna, M. S. Seo, J. Lee, J. Kim, T. Kitagawa, E. Münck, L. Que and W. Nam, J. Am. Chem. Soc., 2005, 127, 12494–12495 CrossRef CAS PubMed.
- L. Ge, C. C. Han and J. Liu, Appl. Catal., B, 2011, 108, 100–106 CrossRef.
- X. F. Hu, T. Mohamood, W. H. Ma, C. C. Chen and J. C. Zhao, J. Phys. Chem. B, 2006, 110, 26012–26015 CrossRef CAS PubMed.
- L. Yuan, M. Q. Yang and Y. J. Xu, Nanoscale, 2014, 6, 6335–6345 RSC.
- C. H. An, S. Peng and Y. G. Sun, Adv. Mater., 2010, 22, 2570–2575 CrossRef CAS PubMed.
- C. L. Schmidt, R. E. Dinnebier and M. Jansen, Solid State Sci., 2009, 11, 1107–1113 CrossRef CAS.
- J. G. Yu, Q. J. Xiang and M. H. Zhou, Appl. Catal., B, 2009, 90, 595–602 CrossRef CAS.
- P. Zhou, J. G. Yu and Y. X. Wang, Appl. Catal., B, 2013, 142, 45–53 CrossRef.
- G. Q. Tan, L. L. Zhang, H. J. Ren, S. S. Wei, J. Huang and A. Xia, ACS Appl. Mater. Interfaces, 2013, 5, 5186–5193 CAS.
- M. L. Guan, C. Xiao, J. Zhang, S. J. Fan, R. An, Q. M. Cheng, J. F. Xie, M. Zhou, B. J. Ye and Y. Xie, J. Am. Chem. Soc., 2013, 135, 10411–10417 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: EDS and TEM. See DOI: 10.1039/c5ra19379f |
| This journal is © The Royal Society of Chemistry 2015 |