Viscoelastic system from mixing cetyltrimethylammonium bromide and poly(styrene-co-methacrylic acid) in aqueous solution†
Abstract
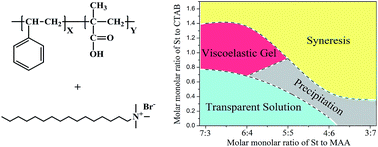
A viscoelastic system was developed by forming hybrid wormlike micelles with poly(styrene-co-methacrylic acid) (P(St-co-MAA)) and cetyltrimethylammonium bromide (CTAB) in aqueous solution. The molar monomer ratio of St to MAA in the copolymer and the mixing ratio (r) of P(St-co-MAA) to CTAB were the two key factors to obtain the viscoelastic system. Phase behaviors of P(St-co-MAA)/CTAB aqueous solutions as the functions of the monomer ratio in the copolymer, as well as the mixing ratio were investigated. It was found that there was no phase separation in any mixing ratios as monomer ratio of 6 : 4 and 7 : 3. On the other hand, the biggest viscosity was observed as the molar ratio of phenyl group in copolymer to CTAB was close to 1, rather than at charge stoichiometry between MAA and CTA+. It is found that as r between 1.0 and 1.24 and the monomer ratio of St to MAA at 7 : 3, the mixing system had the largest viscosity. The effects of the total concentration of the mixture system, the salt concentration, the temperature, and the shear rate on the viscosity were studied in detail. The experimental results showed that the complex system had excellent thickening property even the copolymer concentration as low as 0.90% (w/v), good electrolyte tolerance as inorganic salt concentration lower than 1.8 M, and acceptable heat-resistance at room temperature. The complex system had a potential application as the thickeners in various fields.

 Please wait while we load your content...
Please wait while we load your content...