High quantum-yield luminescent MoS2 quantum dots with variable light emission created via direct ultrasonic exfoliation of MoS2 nanosheets†
Jing-Yuan
Wu
ab,
Xiao-Yang
Zhang
ab,
Xiao-Dan
Ma
ab,
Yun-Ping
Qiu
a and
Tong
Zhang
*ab
aSchool of Electronic Science and Engineering, Southeast University, Key Laboratory of Micro-Inertial Instrument and Advanced Navigation Technology, Ministry of Education, Nanjing, 210096, People's Republic of China. E-mail: tzhang@seu.edu.cn
bSuzhou Key Laboratory of Metal Nano-Optoelectronic Technology, Suzhou Research Institute of Southeast University, Suzhou, 215123, People's Republic of China
First published on 27th October 2015
Abstract
We report a sonication combined with ion intercalation method in an alkaline environment to fabricate MoS2 quantum dots (QDs), with the quantum-yield of the QDs increasing from 0.99% to 4.84% with the additive sodium hydroxide. The QDs displaying variable photoluminescence emission were systematically studied and applied to the bio-imaging field.
MoS2 has received enormous interest due to its direct band gap property and photoluminescence (PL) behaviour when exfoliated to a single-layer.1 Zero-dimensional (0D) MoS2 quantum dots (QDs) maintain the excellent electrocatalytic properties of two-dimensional (2D) MoS2, which could be applied to the hydrogen evolution reaction and analytical chemistry.2–4 They also exhibit improved luminescence efficiency as they have higher specific surface area compared to the 2D counterpart.5 Moreover, some specially prepared MoS2 QDs display interesting optical properties, for example, their fluorescence bands can be tuned in the visible spectrum by only changing the excitation wavelengths without varying QDs' size or chemical composition,6–8 which is quite different from the traditional fluorophores and therefore has recently become a new research direction.4,6 This kind of excitation-dependent fluorescent QDs (EDFQDs) also includes some of the reported graphene QDs and carbon dots, which possess a strong edge effect and size effect, low cytotoxicity, excellent solubility and chemical inertia.8–11 It is well known that, under the excitation of short wavelengths, the conventional polydisperse fluorophores including organic dyes and inorganic QDs emit white light.12,13 While at the same excitation condition, the polydisperse EDFQDs still emit monochromatic light rather than white light. The PL spectra of the optimized EDFQDs exhibit relatively narrow single emitting band, not multiple peaks or one broad band under high-energy irradiation.4,6,7 This newly observed PL behaviour has been inspiring researchers' interests in EDFQDs, however, their excitation-dependent PL mechanism is still under debate.3,14,15 Some researchers considered that the variable PL behaviour resulted from solvent effect not the QDs themselves,16 which needs further research and analysis.
Currently, the reported synthesis methods include hydrothermal synthesis, electrochemically induced Fenton reaction and Li intercalation exfoliation.2,16,17 However, the biggest challenges are still focused on how to improve the quantum-yield of MoS2 QDs. Until now, the reported highest quantum yield is only 2.6%.2
Herein, we showed that the quantum-yield of MoS2 reached 4.84% via the sonication combined with ions intercalation in an alkaline environment. The synthesis method was facile, green and operated at room temperature. Moreover, we systematically studied the optical properties of the QDs, especially their distinct multicolour PL emission ranging from blue to red, and further analyzed the PL origin with the solvent effect excluded. Finally, we utilized the MoS2 QDs with tunable optical properties to image lung cells, coliform bacteria and Staphylococcus aureus, respectively, which showed that the MoS2 QDs of high quantum-yield have great potential applying to the bio-imaging field.
The schematic illustration of the synthesis procedure via ultrasonic exfoliation is shown in Fig. 1. There are two critical steps during the process. The first one is ultrasonic exfoliation process,18–20 in which ultrasonic waves generate vast swarms of vacuum bubbles. The collapses of bubbles lead to high temperature, pressure and fast liquid jets locally, which brings in hydrodynamic shear-force and deagglomeration. At the same time, due to the presence of the standing wave in the solution, small MoS2 nanostructures could be peeled off duly from the bulk MoS2 materials.6,21 Secondly, adding alkali metal ions during the latter process can promote exfoliate more nanosheets and increase the yield.22 By combining the two processes, more 2D nanosheets can be obtained, at the same time, 0D QDs with smaller lateral size can also be obtained in a large scale. To extract the QDs from the solution, we used centrifugation followed by filtration process.
To understand the above forming process of MoS2 QDs, we measured the transmission electron microscopy (TEM) images of the samples prepared at different synthesis stages. In the initial stage, MoS2 nanosheets with the lateral size of about several hundred nanometers were obtained after sonication in N-methylpyrrolidone (NMP) for three hours as shown in the TEM image (Fig. 2(a)). The selected area electron diffraction (SAED) pattern in the inset of Fig. 2(a) indicates that the exfoliated MoS2 was of six-fold symmetry, which demonstrated that the phase of MoS2 is 2D hexagonal semiconducting.23 At the same time, three hours' sonication resulted in the formation of a small amount of MoS2 QDs as shown in Fig. 2(b). When further increasing the sonication time to five hours and adding alkali metal ions during the process to help improve the efficiency of exfoliation, nanosheets with much smaller lateral size of dozens of nanometers were observed as shown in Fig. 2(c). The SAED pattern of the nanosheets obtained after five hours' sonication changed to a faint ring connected by lattices (inset in Fig. 2(c)). Meantime, the proportion of QDs in the sample increased significantly as shown in Fig. 2(d). To completely separate the QDs from the mixed solution, 0.22 μm microporous membranes were utilized to filter the solution for several times. Finally, uniformly dispersed MoS2 QDs were obtained as shown in Fig. 2(e). The HRTEM image in the inset of Fig. 2(e) shows that the lattice spacing of one of as-prepared MoS2 QDs was 0.23 nm, which is matched with (103) lattice plane of MoS2. It also indicates that the QD had good crystallinity. According to the size distribution (Fig. 2(f)), we see that the size of as-prepared QDs ranged from 1.5 nm to 7 nm with an average value of 3.5 nm (the size was statistically calculated from more than 200 QDs in the TEM image of Fig. 2(e)). The SAED pattern in Fig. 2(e) shows that the diffraction pattern changed to an aureole completely. The above TEM images illustrate the forming process of QDs clearly which is consistent with the introduced synthesis mechanism. We concluded that sonication combined with ions intercalation led to the significant reduction of lateral size of MoS2, at the meantime, the structure of MoS2 gradually changed from monocrystalline (2D structure) to polycrystalline (0D structure). In addition, we tried to prepare MoS2 QDs in two other solvents, N,N-dimethylformamide (DMF) and 45 vol% ethanol/water solution. The results indicate that QDs could also be obtained, however, the prepared QDs in the two solvents were obviously less and of low crystallinity as shown in Fig. S1.† The reason is that the surface energy of NMP is more closed to that of MoS2, passivating the prepared QDs into isolated forms and making the QDs stable in the solution, which has also been demonstrated in other literatures.18,24
We also measured the optical absorbance of the three solutions which had been characterized by TEM before to show their optical properties. Fig. 3 exhibits the UV-vis absorbance spectra of the solution sonicated for 3 hours and 5 hours before and after filtration, respectively. The insets in Fig. 3 are corresponding optical photographs of the above solutions, which show that the colour of the solution turned from black to yellowish. The excitonic peak at 670 nm and 610 nm are arising from the K point of the Brillouin zone in 2D MoS2 nanosheets with large lateral dimension.23 The peaks at ∼400 nm and 450 nm are attributed to direct band edge recombination. While in terms of the solution sonicated for 5 hours, the optical absorbance peak at 400 nm and 450 nm showed a slight blue shift. Moreover, the characteristic peaks of large MoS2 nanosheets at 610 nm and 670 nm disappeared after filtration, indicating that the 2D nanosheets were totally removed from the solution. Meantime, the optical absorption of QDs exhibited an apparent blue-shift compared to the nanosheets due to the quantum size effect.23 We noted that the changes of the optical absorbance spectra of these three solutions are well matched with the proposed forming process of MoS2 QDs shown in the previous TEM results in Fig. 2, demonstrating that only MoS2 QDs were obtained finally.
We further explored to improve the quantum-yield of MoS2 QDs through a series of experiments. Surprisingly, we found that both ions intercalation and the alkaline environment contributed to the improvement of the quantum-yield. We measured the PL intensity of solutions with the additive of sodium chloride (NaCl), sodium carbonate (Na2CO3) and sodium hydroxide (NaOH), respectively. The solution without any additives was also measured for comparison. We observed that the PL intensities of solution with additives were both greater than that without additives (Fig. S2(a)†). Moreover, the solution adding NaOH had the highest PL intensity at the excitation of 440 nm. We further measured the absolute quantum-yield of MoS2 QDs solution without and with NaOH additive, respectively. The result showed that the quantum-yield increased from 0.99% to 4.84% at the excitation of 440 nm. The quantum-yield of QDs solution with NaOH excited at other wavelengths was estimated in the range of 0.5–4.5% according to the ratio of integral areas. The mechanism for enhanced PL intensity with the additives is analyzed as follows. On one hand, intercalated ions including Li+ and Na+ could improve the exfoliation efficiency of 2D nanosheets and manipulate the physical property,25 especially luminescence property.22,26 On the other hand, the intensity of PL increases in the alkaline environment, which indicates that the surface states may also play an important role in the luminescence of MoS2 QDs.15
The prepared MoS2 QDs solution displayed interesting variable light emitting property. Fig. 4(a) shows the PL spectra of MoS2 QDs solution adding NaOH excited under different wavelengths ranging from 320 nm to 520 nm. The emission spectra of MoS2 QDs exhibited an excitation-dependent feature. With the increase of excitation wavelength, the emission band gradually red-shifted and its intensity peaked at 440 nm. In addition, we observed the emission peak kept single under different excitations, which means the emitting light of such QDs is more pure.3 The QDs solution adding lithium hydroxide (LiOH) exhibited the same PL behaviour as shown in Fig. S2(b).† The full width at half maximum (FWHM) is about 100 nm which approximates to that of fluorescent organic dyes. However, the excitation spectra of MoS2 QDs is much broader than that of organic dyes.27 What distinguishes the MoS2 QDs from traditional inorganic fluorophores is that MoS2 QDs emit monochromatic light which covers the visible spectrum ranging from blue to red under different excitations. Though the value of FWHM of MoS2 is larger than that of traditional semiconductor QDs such as cadmium sulfide (CdS) and cadmium selenide (CdSe), its unique variable emission feature makes it promising for many fields, such as printing patterns, detecting biomoleculars and metal ions.28–30
To analyze the reasons leading to the PL behaviour of MoS2 QDs solution, we measured the lifetime of the QDs solution as shown in Fig. S3.† The measurement result indicates that there are different species so that the existence of trap states may contribute to the special emission of MoS2 QDs which has also been observed in graphene QDs.31 Some researchers also regarded the presence of polydisperse MoS2 QDs with different sizes as the cause for excitation-dependent emission3 which has also been confirmed by Fig. 2(d). It is noted that commonly reported semiconductor QDs emit white light by mixing QDs of different sizes elaborately.13 However, the MoS2 QDs of different sizes emitted monochrome light as same as other types of EDFQDs. The reason is perhaps that the light emission of EDFQDs is of strict selectivity of excitation wavelength so that it has relatively narrow emission spectrum even at the excitation of photons of high energy. Moreover, the giant spin–orbit coupling of the QDs may also contribute to the multicolour emission similar to the reported WS2 QDs.32
In terms of the variable light emission phenomenon, some researchers considered that it may be related to the PL of the solvent.16 Indeed the reported PL experiments based on MoS2 QDs were constructed in solution state and the solvent effect was not excluded.2–4,6 Hence, excluding the solvent effect is absolutely essential to demonstrate the PL behaviour is originated from MoS2 QDs. We investigated the PL property of dried MoS2 QDs in situ directly using confocal fluorescent microscope as shown in Fig. 4(b)–(d). After evaporating the solvent, the dried QDs still exhibited strong fluorescence under the irradiation of laser. Moreover, the QDs displayed obvious variable light emission for the same area which contained the gathered QDs under different excitation wavelengths. We observed three emission colours: blue, green and red emissions under 405 nm, 488 nm and 552 nm excitations, respectively, in which the green emission was the strongest. It is noted that the differences among PL intensity under the three excitations are consistent with the measured PL spectra of solution (Fig. 4(a)). According to the experimental result, we concluded that the MoS2 QDs still display remarkable variable light emission property with the solvent effect excluded, which provides a direct experimental evidence for further analysing the PL mechanism in the future. In addition, combing such EDFQDs with metal nano-films with tunable plasmon resonance properties may be promising for novel optoelectronic devices.33,34
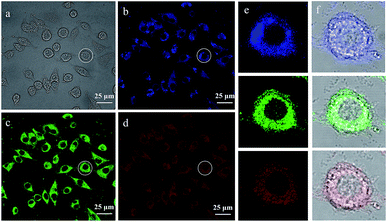
Taking advantage of the multicolour PL property of MoS2 QDs, we continued to explore their application in bio-imaging field via the confocal fluorescence microscope. The confocal images of MoS2 QDS internalized by the lung cells are shown in Fig. 5. Observation of the bright areas inside the cells (Fig. 5(b)–(d)) indicates that MoS2 QDs permeated into the cells successfully without penetrating the nuclei. Thus, the genetic sequence would not be disrupted, confirming the low toxicity of the probes. Fig. 5(e) is the magnified fluorescent image of the single cell marked in Fig. 5(a), and the corresponding merged images of fluorescent and bright field are shown in Fig. 5(f), in which we observed the whole morphology of cell clearly and distinguished the local structure imaged by MoS2 QDs at the same time. Apart from the lung cells, coliform bacteria and Staphylococcus aureus without internalization could also be imaged with MoS2 QDs (Fig. S4†). Therefore, the QDs with good bio-compatibility and unique PL property displaying single-peak variable emission bands covering the entire visible wavelength range can be used for special applications. For example, utilizing their excitation-dependent emission property, one can image living cells under multiple wavelengths avoiding fluorescence interference. In addition, through combining MoS2 QDs and organic dyes with different analytes located at different areas, respectively, MoS2 QDs and organic dyes can emit light of different colours at the same time under the excitation of appropriate wavelength, making it possible to distinguish hybrid analytes at different areas.
Conclusions
In conclusion, we have developed a sonication combined with ions intercalation method in the alkaline environment for fabricating high-yield MoS2 QDs without any heat treatment. We explored a variety of materials to improve the PL intensity of the QDs solution, in which NaOH was found to be the best. The absolute quantum yield of MoS2 QDs with NaOH additive reached 4.84% at the excitation of 440 nm which is nearly double of the previously reported highest quantum yield of MoS2 QDs. Moreover, the quantum yield of QDs at other excitations was also estimated in the range of 0.5–4.5%, indicating the MoS2 QDs display relatively high-efficiency PL emission at the excitation of a broad spectrum. We also showed the variable PL emission of MoS2 QDs solution whose relatively narrow-band emission covered the entire visible spectrum, making MoS2 QDs distinguish from traditional fluorophores. We further investigated the PL behaviour of dried MoS2 QDs with the solvent effect excluded, which confirms that the PL behaviour of variable emission is originated from MoS2 QDs. Finally, we took a variety of cell imaging experiments and spectroscopic analysis, which showed that currently reported MoS2 QDs with a series of advantages, such as high quantum-yield, remarkable narrow-band light emission property and good biocompatibility, have great application prospective in biological cell imaging and component analysis. This unique PL property also make such MoS2 QDs promising for optoelectronic field, being used to fabricate novel multicolour display devices, sensors and lasers in the future.Acknowledgements
This work is supported by NSFC under grant number 61307066, Doctoral Fund of Ministry of Education of China under grant number 20130092120024, Natural Science Foundation of Jiangsu Province under grant number BK20130630, the Fundamental Research Funds for the Central Universities and Graduate Innovation Program of Jiangsu Province under grant number KYLX_0126, Innovation fund of School of Electronic science and engineering, Southeast University under grant number 2242015KD006.Notes and references
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- Y. Wang and Y. Ni, Anal. Chem., 2014, 86, 7463–7470 CrossRef CAS PubMed.
- D. Gopalakrishnan, D. Damien and M. M. Shaijumon, ACS Nano, 2014, 8, 5297–5303 CrossRef CAS PubMed.
- D. Gopalakrishnan, D. Damien, B. Li, H. Gullappalli, V. K. Pillai, P. M. Ajayan and M. M. Shaijumon, Chem. Commun., 2015, 51, 6293–6296 RSC.
- A. Splendiani, L. Sun, Y. Zhang, T. Li, J. Kim, C.-Y. Chim, G. Galli and F. Wang, Nano Lett., 2010, 10, 1271–1275 CrossRef CAS PubMed.
- V. Štengl and J. Henych, Nanoscale, 2013, 5, 3387–3394 RSC.
- S. K. Cushing, M. Li, F. Huang and N. Wu, ACS Nano, 2013, 8, 1002–1013 CrossRef PubMed.
- S. Zhu, J. Zhang, C. Qiao, S. Tang, Y. Li, W. Yuan, B. Li, L. Tian, F. Liu and R. Hu, Chem. Commun., 2011, 47, 6858–6860 RSC.
- Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang and H. Wang, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- R. Liu, D. Wu, S. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., 2009, 121, 4668–4671 CrossRef.
- Y. Li, Y. Hu, Y. Zhao, G. Shi, L. Deng, Y. Hou and L. Qu, Adv. Mater., 2011, 23, 776–780 CrossRef CAS PubMed.
- Q. Dai, C. E. Duty and M. Z. Hu, Small, 2010, 6, 1577–1588 CrossRef CAS PubMed.
- C.-C. Shen and W.-L. Tseng, Inorg. Chem., 2009, 48, 8689–8694 CrossRef CAS PubMed.
- X. Zhang, Z. Lai, Z. Liu, C. Tan, Y. Huang, B. Li, M. Zhao, L. Xie, W. Huang and H. Zhang, Angew. Chem., Int. Ed., 2015, 54, 5425–5428 CrossRef CAS.
- D. Pan, J. Zhang, Z. Li, C. Wu, X. Yan and M. Wu, Chem. Commun., 2010, 46, 3681–3683 RSC.
- H. D. Ha, D. J. Han, J. S. Choi, M. Park and T. S. Seo, Small, 2014, 10, 3858–3862 CrossRef CAS PubMed.
- B. L. Li, L. X. Chen, H. L. Zou, J. L. Lei, H. Q. Luo and N. B. Li, Nanoscale, 2014, 6, 9831–9838 RSC.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. de and R. J. Smith, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- J.-Y. Wu, M.-N. Lin, L.-D. Wang and T. Zhang, J. Nanomater., 2014, 2014, 1–6 Search PubMed.
- L. Grande, V. T. Chundi, D. Wei, C. Bower, P. Andrew and T. Ryhänen, Particuology, 2012, 10, 1–8 CrossRef CAS.
- H. Li, X. He, Y. Liu, H. Huang, S. Lian, S.-T. Lee and Z. Kang, Carbon, 2011, 49, 605–609 CrossRef CAS.
- G. S. Bang, K. W. Nam, J. Y. Kim, J. Shin, J. W. Choi and S.-Y. Choi, ACS Appl. Mater. Interfaces, 2014, 6, 7084–7089 CAS.
- Y. Wang, J. Z. Ou, S. Balendhran, A. F. Chrimes, M. Mortazavi, D. D. Yao, M. R. Field, K. Latham, V. Bansal and J. R. Friend, ACS Nano, 2013, 7, 10083–10093 CrossRef CAS.
- S. Chandra, S. H. Pathan, S. Mitra, B. H. Modha, A. Goswami and P. Pramanik, RSC Adv., 2012, 2, 3602–3606 RSC.
- D. Wei, L. Grande, V. Chundi, R. White, C. Bower, P. Andrew and T. Ryhänen, Chem. Commun., 2012, 48, 1239–1241 RSC.
- J. Z. Ou, A. F. Chrimes, Y. Wang, S.-Y. Tang, M. S. Strano and K. Kalantar-zadeh, Nano Lett., 2014, 14, 857–863 CrossRef CAS PubMed.
- T. Zhang and F. Shan, J. Nanomater., 2014, 2014, 1–15 Search PubMed.
- R.-Z. Li, A. Hu, D. Bridges, T. Zhang, K. D. Oakes, R. Peng, U. Tumuluri, Z. Wu and Z. Feng, Nanoscale, 2015, 7, 7368–7377 RSC.
- X. Zhang, X. Ma, F. Dou, P. Zhao and H. Liu, Adv. Funct. Mater., 2011, 21, 4219–4227 CrossRef CAS.
- A. K. Yetisen, Y. Montelongo, M. M. Qasim, H. Butt, T. D. Wilkinson, M. J. Monteiro and S. H. Yun, Anal. Chem., 2015, 87, 5101–5108 CrossRef CAS PubMed.
- D. B. Shinde and V. K. Pillai, Chem.–Eur. J., 2012, 18, 12522–12528 CrossRef CAS PubMed.
- L. Lin, Y. Xu, S. Zhang, I. M. Ross, A. C. Ong and D. A. Allwood, ACS Nano, 2013, 7, 8214–8223 CrossRef CAS PubMed.
- K. Isozaki, T. Ochiai, T. Taguchi, K.-I. Nittoh and K. Miki, Appl. Phys. Lett., 2010, 97, 221101 CrossRef.
- X.-Y. Zhang, A. Hu, T. Zhang, W. Lei, X.-J. Xue, Y. Zhou and W. W. Duley, ACS Nano, 2011, 5, 9082–9092 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supplementary figures. See DOI: 10.1039/c5ra19201c |
| This journal is © The Royal Society of Chemistry 2015 |