Catalytic properties of γ-Al2O3 supported Pt–FeOx catalysts for complete oxidation of formaldehyde at ambient temperature
Weiyi Cuiab,
Xiaoling Yuana,
Ping Wua,
Bin Zhenga,
Wenxiang Zhang*a and
Mingjun Jia*a
aKey Laboratory of Surface and Interface Chemistry of Jilin Province, College of Chemistry, Jilin University, 130021 Changchun, China. E-mail: jiamj@jlu.edu.cn; Fax: +86-431-85168420; Tel: +86-431-85155390
bKey Laboratory of Chemical Cleaner Production Technology of Jilin Province, Jilin Institute of Chemical Technology, 132022 Jilin, China
First published on 3rd December 2015
Abstract
A series of γ-Al2O3 supported Pt–FeOx catalysts (Pt–FeOx/Al2O3) with different Fe/Pt atom ratios were prepared, and their catalytic properties were investigated in the oxidation of formaldehyde. It was found that the catalytic activities of Pt–FeOx/Al2O3 catalysts are varied with the change of Fe/Pt ratios. Among them, the sample with a Fe/Pt ratio of 1.0 exhibits the highest activity, which can efficiently convert formaldehyde to CO2 at ambient temperature. The catalytic activity of the Pt–FeOx/Al2O3 catalyst can be further improved by the addition of water vapor into the feed stream. A variety of characterization results showed that both Pt nanoparticles and FeOx species are highly dispersed on the surface of the γ-Al2O3 support. Changing Fe/Pt ratios could influence the chemical states and the redox properties of Pt and Fe species. The catalysts with appropriate Fe/Pt ratios have more accessible active sites, i.e., the Pt–O–Fe species, which are located at the boundaries between FeOx and Pt nanoparticles, thus showing high activity for the oxidation of formaldehyde under ambient conditions.
1 Introduction
Recently, significant efforts have been made to study the removal of the toxic air pollutant formaldehyde (HCHO) for satisfying the environmental regulations and human health needs.1–4 Catalytic oxidation of HCHO by oxygen has been considered as one of the most effective approaches, which only yields harmless products of CO2 and H2O.5–14 It is highly desirable to achieve complete oxidation of HCHO at ambient temperature for saving energy. So far, a variety of supported noble-metal (Au, Pt, Ag, Pd, etc.) catalysts have shown unusual low-temperature activity for complete oxidation of HCHO.11–14 Among them, Pt-based catalysts have drawn more attention for its superior catalytic activity and excellent stability, and several highly active supported Pt catalysts for room-temperature oxidation of HCHO have already been obtained by selecting appropriate supports and preparation methods, including Pt/TiO2,1,12,15–20 Pt/MnOx–CeO2,21 Pt/Fe2O3,22 Pt/SiO2,23 Pt/MnO2,24 Pt/ferrihydrite.25As a common support material, commercial Al2O3 has been widely used to prepare supported noble-metal catalysts due to its great advantages, such as low cost, thermal and chemical stability, high surface area and so on.26 However, early literatures showed that the catalytic activities of the Al2O3 supported Pt catalysts are unsatisfied for low-temperature oxidation of HCHO or CO.27,28 Recently, by adjusting the structure and surface property of Al2O3 support, a few relatively active Al2O3 supported Pt catalysts could also be prepared.29–31 For instance, M. Jaroniec and co-workers reported that hierarchically macro–mesoporous Al2O3 and nanostructured AlOOH supported Pt catalysts are active for the oxidative decomposition of HCHO at room-temperature.30,31 Currently, it is still a very interesting subject to design highly active supported Pt catalysts by using commercial available Al2O3 as support material for low-temperature oxidation of HCHO.
Previously, our group has already carried out some work on the studies of low-temperature oxidation of HCHO over iron oxides or fumed silica supported Pt catalysts.22,23 The Pt/Fe2O3 catalysts, prepared by colloid-deposition method and calcined at appropriate temperature, exhibited very high catalytic activity for the oxidation of HCHO or CO at ambient temperature.22,32,33 Herein, we tried to use commercial Al2O3 material as support to prepare supported Pt–FeOx catalysts (Pt–FeOx/Al2O3), and their catalytic activities were investigated in the complete oxidation of HCHO. It was found that highly active catalyst can be obtained by adjusting the Fe/Pt atom ratios of the Pt–FeOx/Al2O3 catalysts, and their catalytic activity can be further improved by the addition of water vapor into the feed stream. Moreover, a variety of characterization means were carried out to study the physico-chemical properties of the Pt–FeOx/Al2O3 catalysts, and to understand the nature of the active sites for low-temperature oxidation of HCHO.
2 Experimental section
2.1 Catalyst preparation
The Pt–FeOx colloids with different Fe/Pt atom ratios were prepared on the basis of a previously reported procedure for preparing Pt colloids.22 The Pt–FeOx/Al2O3 catalysts were obtained by supporting Pt–FeOx colloids on the commercial Al2O3 through colloid-deposition route.32,34 Typically, a glycol solution of NaOH was added into a glycol solution of H2PtCl6 under stirring for 1 h, and to form the colloidal solution by heating at 140 °C under the protection of Ar. Then, a glycol solution of FeCl2 was added to the solution of Pt colloid. After cooling down to 80 °C, Al2O3 was added into the Pt–FeOx colloid solution under stirring, and the mixed slurry was then kept statically for 12 h to achieve the deposition process. The solid was isolated and washed thoroughly with distilled water. The filtered solid products were dried at 100 °C for 10 h and calcined at 200 °C in a flow of 20% O2/Ar for 2 h. The resulting catalysts are herein denoted as Pt–nFeOx/Al2O3, where n represents the molar ratio of Fe/Pt (n = 0, 0.3, 1.0, 2.5 and 4.0). The loading of Pt is approximately 2% for each catalyst.2.2 Catalyst characterization
The X-ray diffraction (XRD) analyses of the catalysts were carried out using a D/Max-rA X-ray diffractometer operated at 30 kV and 40 mA employing nickel-filtered Cu Kα radiation. Transmission electron microscopy (TEM) images and the high-angle annular dark field STEM (HAADF-STEM) images were obtained using a FEI Tecnai F20 EM operated at 200 kV and equipped with an energy-dispersive spectroscopy analyzer. The X-ray photoelectron spectra (XPS) measurements were carried out on an ESCALAB250 X-ray photoelectron spectrometer with Al Kα radiation as excitation source. H2–O2 titration experiments were carried out on a Builder PCA-1200 Pulse Chemisorption System. Prior to chemisorption, the catalyst (0.1 g) was pretreated in flowing argon for 1 h at 100 °C, and then reduced under pure H2 (50 mL min−1) at 200 °C for 1 h. After purging with Ar (50 mL min−1), the sample was oxidized under pure O2 (50 mL min−1) at 400 °C for 2 h and then purged under Ar (50 mL min−1). After cooling down to 25 °C, H2 pluses were injected into the carrier gas and the whole process was monitored by a thermal conductivity detector. The XPS spectra were corrected by adjusting the C 1s peak to a position of 284.6 eV. Temperature programmed reduction by H2 measurements (H2-TPR) were carried out using an adsorption instrument equipped with a TCD. The samples were loaded and pretreated with Ar at 100 °C for 30 min. The H2-TPR experiment was performed under the mixture of 5% H2 in N2 flow (30 mL min−1) over 20 mg of catalyst at a heating rate of 10 °C min−1.2.3 Catalytic test
For the HCHO oxidation tests, 0.1 g of solid catalyst sample (40–60 mesh) was loaded in a quartz tube reactor. The gas mixture consisted of HCHO 300–400 ppm, 20 vol% O2, and a certain amount of water vapor (with relative humidity of 0–80%) balanced by N2. Gaseous HCHO was generated by flowing N2 coming from a mass-flow controller through aqueous formaldehyde in an incubator. Products and reactants were analyzed by Techcomp GC-7900 gas chromatograph equipped with TCD detector, and the catalytic activity of the Pt–FeOx/Al2O3 catalysts was evaluated by the conversion of formaldehyde to CO2.3 Results and discussion
3.1 Characterization of the catalysts
XRD patterns of the four Pt–nFeOx/Al2O3 catalysts as well as the support of γ-Al2O3 are shown in Fig. 1. For γ-Al2O3 support, some weak diffraction peaks appeared at 2θ = 37.6, 45.8, 66.8 (JCPDS no. 29-0063), which could be associated with the presence of γ-Al2O3 phase. For the four Pt–nFeOx/Al2O3 catalysts, it is worth to note that the characteristic diffraction peaks of platinum species and crystalline phases of iron oxide do not appear, indicating that both Pt species and Fe species should be highly dispersed on the surface of Al2O3 support. | ||
| Fig. 1 X-ray diffraction patterns for Pt–FeOx/Al2O3 catalysts. (a) Pt–0.3FeOx/Al2O3; (b) Pt–1.0FeOx/Al2O3; (c) Pt–2.5FeOx/Al2O3; (d) Pt–4.0FeOx/Al2O3; (e) γ-Al2O3. | ||
The morphology of a representative catalyst of Pt–1.0FeOx/Al2O3 was investigated with HRTEM, HAADF-STEM and EDX mapping (Fig. 2). It is found that the platinum nanoparticles, observed as dark spots in HRTEM images and bright spots in HAADF-STEM images (Fig. 2a and c), are homogeneously dispersed on the surface of Al2O3 supports. The lattice fringes of d spacing value (0.224 nm) match well with the crystallographic plane of Pt (111), and the lattice fringes of d spacing value (0.239 nm) correspond to the (311) plane of γ-Al2O3. The average size of Pt nanoparticles is around 2.2 nm. These results suggest that no obvious agglomeration of platinum nanoparticles takes place on the surface of the γ-Al2O3. Besides, no large particles of iron oxide can be observed in the whole detected region even in high resolution measurement (Fig. 2a–c). The resulting EDX mapping images clearly indicate that iron oxides should be highly dispersed on the surface of γ-Al2O3 support in the form of very small particles (Fig. 2d).
As shown in Table 1, the Pt dispersion of the four Pt–FeOx/Al2O3 catalysts was determined by the H2–O2 titration experiments. It was found that the Pt dispersion on the Pt–1.0FeOx/Al2O3 is 38.8%, much higher than that of other catalysts with different Fe/Pt ratios. The relatively low dispersion of the Pt–2.5FeOx/Al2O3 and Pt–4.0FeOx/Al2O3 catalysts might be caused by the fact that the excess FeOx species may cover quite amount of Pt nanoparticles during the preparation process for the formation of Pt–FeOx colloids.
| Catalysts | Mol ratio of Pt/Fe/Al | Pt dispersion (%) |
|---|---|---|
| Pt–0.3FeOx/Al2O3 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
24.6 |
| Pt–1.0FeOx/Al2O3 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
38.8 |
| Pt–2.5FeOx/Al2O3 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
20.0 |
| Pt–4.0FeOx/Al2O3 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.0 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
17.2 |
The Pt 4d5/2 XPS spectra of the Pt–FeOx/Al2O3 catalysts are shown in Fig. 3. The deconvolution of the Pt 4d5/2 photopeak provides a main peak at 314.7 eV and a weak peak at 317.3 eV, which can be assigned to metallic Pt0 and oxidized Pt2+ species, respectively.35–37 For all the four catalysts, the chemical states of Pt species are basically consistent with each other, which are composed of a majority of Pt0 species and a small portion of Pt2+ species. Most of the Pt0 species should be formed by the reduction of oxidized Pt species with glycol during the heating process for preparing Pt colloids. Notably, the signal intensity of Pt 4d peaks for Pt–2.5FeOx/Al2O3 and Pt–4.0FeOx/Al2O3 catalysts is much weaker than that of Pt–0.3FeOx/Al2O3 and Pt–1.0FeOx/Al2O3 catalysts, suggesting that the surface concentrations of Pt species decrease gradually with the increase of Fe/Pt atom ratios. These results confirm further that a certain amount of Pt nanoparticles have been covered by the introduced FeOx species, which is particularly serious for the catalysts containing higher loading of FeOx (i.e., Pt–2.5FeOx/Al2O3 and Pt–4.0FeOx/Al2O3). Therefore, changing Fe/Pt ratios of the Pt–FeOx/Al2O3 catalysts may considerably affect the surface concentration of Pt species, which is in good agreement with the related literature results.36
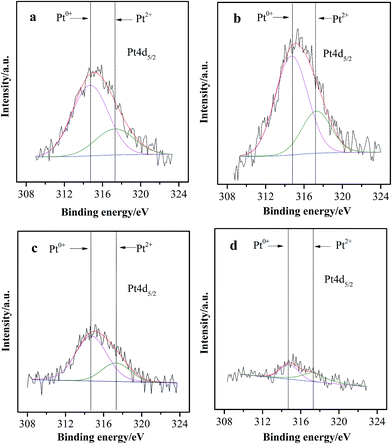 | ||
| Fig. 3 XPS spectra of Pt 4d5/2 for the Pt–FeOx/Al2O3 catalysts (a) Pt–0.3FeOx/Al2O3; (b) Pt–1.0FeOx/Al2O3; (c) Pt–2.5FeOx/Al2O3; (d) Pt–4.0FeOx/Al2O3. | ||
Fig. 4 shows the XPS spectra in the region of the Fe 2p3/2 emission band of the Pt–FeOx/Al2O3 catalysts. On the basis of related literatures,36,38–40 the Fe 2p3/2 signals appeared in the region of 709–713 eV should be mainly assigned to Fe3+ species. The signal intensity of Fe 2p3/2 peak increases gradually with the increase of Fe/Pt atom ratios. Compared with the catalysts containing higher Fe contents (Pt–2.5FeOx/Al2O3 and Pt–4.0FeOx/Al2O3), the binding energies of Fe 2p3/2 for Pt–0.3FeOx/Al2O3 and Pt–1.0FeOx/Al2O3 shift slightly toward lower values. These changes should arise from the interaction between Pt nanoparticles and FeOx species, indicating the formation of Pt–FeOx interface.22,32
 | ||
| Fig. 4 XPS spectra of Fe 2p3/2 region for the Pt–FeOx/Al2O3 catalysts: (a) Pt–0.3FeOx/Al2O3; (b) Pt–1.0FeOx/Al2O3; (c) Pt–2.5FeOx/Al2O3; (d) Pt–4.0FeOx/Al2O3. | ||
Subsequently, the H2-TPR profiles of the four Pt–nFeOx/Al2O3 catalysts and the Fe/Al2O3 catalyst are shown in Fig. 5. The reference sample of Fe/Al2O3 catalyst shows a very broad peak centered at around 200 °C, which can be ascribed to the reduction of dispersed Fe2O3 (to Fe3O4) on the surface of γ-Al2O3.41 For Pt–0.3FeOx/Al2O3 catalyst, the broad reduction peak centered at around 120 °C could be assigned primarily to the reduction of Pt2+ to Pt0 as well as the reduction of the iron oxide intimately contacted with Pt species.22,32
 | ||
| Fig. 5 H2-TPR profiles of Fe/Al2O3 and Pt–FeOx/Al2O3 catalysts. (a) Fe/Al2O3; (b) Pt–0.3FeOx/Al2O3; (c) Pt–1.0FeOx/Al2O3; (d) Pt–2.5FeOx/Al2O3; (e) Pt–4.0FeOx/Al2O3. | ||
For Pt–1.0FeOx/Al2O3, the low-temperature reduction peak shifts to 90 °C accompanied with the increase in peak intensity, which can be mainly attributed to the formation of more easily reduced FeOx species located at the boundary of the Pt and FeOx compared with the Pt–0.3FeOx/Al2O3 catalyst. With further increasing the Fe contents (Pt–2.5FeOx/Al2O3 and Pt–4.0FeOx/Al2O3), the position of the low-temperature reduction peak increases somewhat (up to 100 °C), and its peak area turns to larger. This should be due to the fact the extra FeOx species, which are near from the Pt–O–Fe boundary, can be reduced at slightly higher temperature. Besides, the reduction peaks located at above 200 °C could also be detected for all the Pt–nFeOx/Al2O3 catalysts, which are attributed to the reduction of relatively isolated Fe2O3 (far from Pt nanoparticles) dispersed on the surface of γ-Al2O3.
The above characterization results suggest that both Pt nanoparticles and FeOx species are highly dispersed on the surface of γ-Al2O3 support, and their chemical states and redox properties may be changed somewhat by adjusting the Fe/Pt atom ratios. The presence of relatively strong interaction between Pt nanoparticles and FeOx species can be deduced, which can result in the formation of some interacting Pt and Fe species (i.e., Pt–O–Fe species) on the surface of Al2O3 supports.
3.2 Catalytic activity
The catalytic activities of the Pt–FeOx/Al2O3, as well as the reference samples of Pt/Al2O3, Fe/Al2O3 for HCHO oxidation are shown in Fig. 6. It should be pointed out here that relatively high HCHO concentration (300–400 ppm) was used for easily achieving analysis and making comparison with related literatures.22 Under the tested conditions, both Pt/Al2O3 and Fe/Al2O3 catalysts do not show any activity for HCHO oxidation when the reaction temperatures increase from 25 to 100 °C. As for the four Pt–nFeOx/Al2O3 catalysts, they are all catalytically active for oxidizing HCHO to CO2 and H2O at low temperature. Pt–0.3FeOx/Al2O3 catalyst, which contains very small amount of FeOx, gives a 62% conversion of HCHO at 25 °C, and much higher activity can be obtained over Pt–1.0FeOx/Al2O3, which could achieve complete oxidation of HCHO at 25 °C under the same conditions. With the further increase of Fe loading, obvious decrease in catalytic activity can be detected over Pt–2.5FeOx/Al2O3 and Pt–4.0FeOx/Al2O3 catalysts. The above results suggest that the existence of a certain amount of FeOx plays a key role in forming highly active Pt–FeOx/Al2O3 catalyst for low-temperature oxidation of HCHO.As shown in Fig. 7, the effect of GHSV on the catalytic activity of the Pt–1.0FeOx/Al2O3 catalyst was investigated. At room temperature, 100% HCHO conversion is attained when a GHSV of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm3 g−1 h−1 is used. With the increase of GHSV (or the decrease of contact time), the conversion of HCHO decreases to 89.6% or to 71.3% at the GHSV of 120
000 cm3 g−1 h−1 is used. With the increase of GHSV (or the decrease of contact time), the conversion of HCHO decreases to 89.6% or to 71.3% at the GHSV of 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm3 g−1 h−1 or 180
000 cm3 g−1 h−1 or 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm3 g−1 h−1.
000 cm3 g−1 h−1.
 | ||
| Fig. 7 Formaldehyde catalytic activities of different space velocity for Pt–1.0FeOx/Al2O3 catalyst. Reaction conditions: HCHO 400 ppm, O2 = 20 vol%, N2 balance, RH = 30%. | ||
The effect of humidity on the catalytic property of Pt–1.0FeOx/Al2O3 catalyst was also investigated (Fig. 8). Interestingly, the presence of water actually enhances the HCHO conversion rather than inhibits the catalytic activity of the catalyst. Under dry condition, 76.2% conversion of HCHO is reached at the reaction temperature of 25 °C. Significant improvement in catalytic activity could be observed under wet condition, and nearly 100% HCHO conversion is achieved at 30% or 55% humidity. Slight decrease in catalytic activity (97.2% HCHO conversion) could be detected when the humidity reaches 80%. These results suggest that the presence of suitable amount of water vapor is beneficial for the oxidation of HCHO at low temperature. This feature of the catalyst is very significant since removing HCHO in indoor air is usually charged with different content of water vapor at ambient condition.
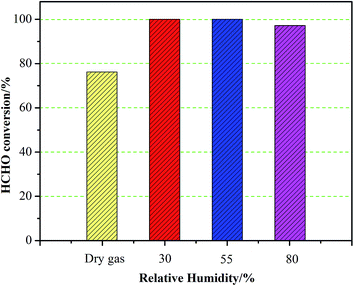 | ||
Fig. 8 Catalytic activities of Pt–1.0 FeOx/Al2O3 catalysts with different relative humidity. Reaction conditions: HCHO 400 ppm, O2 = 20 vol%, N2 balance, GHSV: 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm3 g−1 h−1, temperature: 25 °C. 000 cm3 g−1 h−1, temperature: 25 °C. | ||
3.3 Discussion
Previously, it was reported that supported Pt/Fe2O3 catalysts prepared by colloid deposition route are catalytically active for low-temperature oxidation of CO or HCHO.22,32 The presence of suitable interaction between Pt nanoparticles and iron oxide supports has been revealed, which can result in the formation highly active sites at the interface of these two components. Particularly, it has been proved that the Fe species located at the boundaries between Pt nanoparticles and FeOx supports have shown very strong ability for activating O2 in the supported Pt/FeOx catalysts.42–44The present work shows that the supported Pt–FeOx/Al2O3 catalysts are also quite active for the low-temperature oxidation of HCHO, although both Pt/γ-Al2O3 and Fe/γ-Al2O3 catalysts are inactive under the tested conditions. It can be certainly concluded that the enhanced catalytic activity is related to the interaction between Pt and FeOx. Certainly, the existence of the interaction between Pt nanoparticles and the Al2O3 support cannot be ruled out. However, it seems that such interaction (if exist) does not bring obvious contribution to the high catalytic oxidation activity of the Pt–FeOx/Al2O3 catalysts since the supported Pt/Al2O3 catalyst is nearly inactive under the tested conditions. Therefore, we may propose here that the excellent catalytic activity of Pt–FeOx/Al2O3 should be mainly originated from the formation of interacting Pt and Fe species located at the Pt–FeOx boundary, which are highly distributed on the surface of γ-Al2O3 supports. Compared with the other three catalysts, Pt–1.0FeOx/Al2O3 catalyst exhibits the highest catalytic activity. This might be indicative that more interacting Pt–FeOx species are present on the surface of the catalysts, which are easily accessible for reagents. For the catalysts with higher Fe/Pt atom ratios, the excessive FeOx species may cover a portion of active sites located at the boundaries between FeOx and Pt nanoparticles, thus resulting in the decrease in catalytic activity for the oxidation of formaldehyde.
Recently, the enhanced role of water on the low-temperature oxidation of CO over the supported Pt/FeOx catalysts has been discussed by using in situ DRIFT characterization means.33 It was proposed that introducing water to the reactant stream could be beneficial to the formation of hydroxylated oxide surface through coadsorption of water and oxygen on the surface of the Pt/FeOx catalyst, which might be a critical factor in improving the capability of the supported noble metal catalysts for activating molecular oxygen. For the oxidation of HCHO, it was reported that the presence of moisture may promote the conversion of the intermediates (such as formates) into CO2 and H2O,45–47 thus resulting in the improvement of HCHO oxidation over some supported noble metal catalysts.
In the present work, it was found that the catalytic activity of the Pt–FeOx/Al2O3 catalyst for HCHO oxidation can also been promoted by introducing water vapor into the feed. On the basis of the previous work mentioned above as well as some related literatures,38,48,49 it can be proposed that the introduced H2O molecules are easily transformed to –OH groups by interacting with the FeOx species located at the surface of Pt–FeOx/Al2O3 catalysts. And the resultant –OH groups located at the interface of Pt nanoparticles and FeOx species (like Fe3+–OH–Pt) may act as additional active sites for the oxidation of HCHO or the intermediates (like formates) to produce CO2 and H2O, thus improving the catalytic activity of the Pt–FeOx/Al2O3 catalyst for low-temperature oxidation of HCHO. Besides, the presence of water or surface hydroxyl groups may also be particularly suitable for the adsorption/activation of HCHO through hydrogen bonding interaction, which can certainly play a positive role in improving the catalytic activity of the Pt–FeOx/Al2O3 catalyst. More characterization work is still required to reveal the reaction mechanism of formaldehyde oxidation and the concreted role of water in influencing the catalytic properties of the Pt–FeOx/Al2O3 catalysts.
It should be point out here that the Pt–1.0FeOx/Al2O3 catalyst exhibits quite similar catalytic activity to the previously reported Pt/FeOx catalyst.22 The main advantages of the present work are as follows: using commercial Al2O3 as support would be more important for developing efficient practical catalysts for industry application. Besides, the essence of the interaction between the Pt nanoparticles and the FeOx species can be seen more clearly by comparing the characterization results of the Pt–FeOx/Al2O3 catalysts with that of the Pt/FeOx catalysts.
4 Conclusions
In this study, Pt–FeOx/Al2O3 catalysts with different Fe/Pt atom ratios were prepared by using commercial γ-Al2O3 as support, and their catalytic properties were investigated for the low-temperature oxidation of formaldehyde. A variety of characterization results revealed that the Pt nanoparticles and iron oxides are well dispersed on the surface of γ-Al2O3 support, and there are relatively strong interactions between FeOx and Pt nanoparticles, which are able to adjust the redox properties of the Pt–FeOx/Al2O3 catalysts. By selecting appropriate Fe/Pt ratios, highly active Pt–1.0FeOx/Al2O3 catalyst could be obtained, which could achieve complete oxidation of formaldehyde at ambient conditions. The excellent catalytic property of Pt–1.0FeOx/Al2O3 catalyst should be mainly attributed to the formation of more accessible active sites located at the boundaries between Pt nanoparticles and FeOx species.Acknowledgements
This work was supported by the National Science Foundation of China (Grant No. 20973080 and 21473074).Notes and references
- C. B. Zhang, F. D. Liu, Y. P. Zhai, H. Ariga, N. Yi, Y. C. Liu, K. Asakura, M. Flytzani-Stephanopoulos and H. He, Angew. Chem., Int. Ed., 2012, 51, 9628–9632 CrossRef CAS PubMed.
- P. Zhou, J. G. Yu, L. H. Nie and M. Jaroniec, J. Mater. Chem. A, 2015, 3, 10432–10438 CAS.
- J. H. Zhang, Y. B. Li, L. Wang, C. B. Zhang and H. He, Catal. Sci. Technol., 2015, 5, 2305–2313 CAS.
- X. J. Tang, Y. Bai, A. Duong, M. T. Smith, L. Y. Li and L. P. Zhang, Environ. Int., 2009, 35, 1210–1224 CrossRef CAS PubMed.
- J. Q. Torres, S. Royer, J. P. Bellat, J. M. Giraudon and J. F. Lamonier, ChemSusChem, 2013, 6, 578–592 CrossRef PubMed.
- C. B. Zhang and H. He, Catal. Today, 2007, 126, 345–350 CrossRef CAS.
- X. F. Tang, Y. G. Li, X. M. Huang, Y. D. Xu, H. Q. Zhu, J. G. Wang and W. J. Shen, Appl. Catal., B, 2006, 62, 265–273 CrossRef CAS.
- J. Q. Torres, J. M. Giraudon and J. F. Lamonier, Catal. Today, 2011, 176, 277–280 CrossRef CAS.
- J. Xu, T. White, P. Li, C. H. He and Y. F. Han, J. Am. Chem. Soc., 2010, 132, 13172–13173 CrossRef CAS PubMed.
- Y. C. Huang, H. B. Li, M. S. Balogun, H. Yang, Y. X. Tong, X. H. Lu and H. B. Ji, RSC Adv., 2015, 5, 7729–7733 RSC.
- H. F. Li, N. Zhang, P. Chen, M. F. Luo and J. Q. Lu, Appl. Catal., B, 2011, 110, 279–285 CrossRef CAS.
- L. H. Nie, J. G. Yu, X. Y. Li, B. Cheng, G. Liu and M. Jaroniec, Environ. Sci. Technol., 2013, 47, 2777–2783 CrossRef CAS PubMed.
- L. Ma, D. S. Wang, J. H. Li, B. Y. Bai, L. X. Fu and Y. D. Li, Appl. Catal., B, 2014, 148, 36–43 Search PubMed.
- H. B. Huang, X. G. Ye, H. L. Huang, L. Zhang and D. Y. C. Leung, Chem. Eng. J., 2013, 230, 73–79 CrossRef CAS.
- C. B. Zhang, H. He and K. I. Tanaka, Appl. Catal., B, 2006, 65, 37–43 CrossRef CAS.
- H. B. Huang and D. Y. C. Leung, J. Catal., 2011, 280, 60–67 CrossRef CAS.
- B. T. Liu, C. H. Hsieh, W. H. Wang, C. C. Huang and C. J. Huang, Chem. Eng. J., 2013, 232, 434–441 CrossRef CAS.
- L. H. Nie, P. Zhou, J. G. Yu and M. Jaroniec, J. Mol. Catal. A: Chem., 2014, 390, 7–13 CrossRef CAS.
- H. Y. Chen, Z. B. Rui and H. B. Ji, Ind. Eng. Chem. Res., 2014, 53, 7629–7636 CrossRef CAS.
- L. F. Qi, W. K. Ho, J. L. Wang, P. Y. Zhang and J. G. Yu, Catal. Sci. Technol., 2015, 5, 2366–2377 CAS.
- X. F. Tang, J. L. Chen, X. M. Huang, Y. D. Xu and W. J. Shen, Appl. Catal., B, 2008, 81, 115–121 CrossRef CAS.
- N. H. An, Q. S. Yu, G. Liu, S. Y. Li, M. J. Jia and W. X. Zhang, J. Hazard. Mater., 2011, 186, 1392–1397 CrossRef CAS.
- N. H. An, W. L. Zhang, X. L. Yuan, B. Pan, G. Liu, M. J. Jia, W. F. Yan and W. X. Zhang, Chem. Eng. J., 2013, 215, 1–6 CrossRef.
- Y. Chen, J. H. He, H. Tian, D. H. Wang and Q. W. Yang, J. Colloid Interface Sci., 2014, 428, 1–7 CrossRef CAS PubMed.
- Z. X. Yan, Z. H. Xu, J. G. Yu and M. Jaroniec, Environ. Sci. Technol., 2015, 49, 6637–6644 CrossRef CAS.
- Z. R. Zhang, R. W. Hicks, T. R. Pauly and T. J. Pinnavaia, J. Am. Chem. Soc., 2002, 124, 1592–1593 CrossRef CAS PubMed.
- L. F. Wang, M. Sakurai and H. Kameyama, J. Hazard. Mater., 2009, 167, 399–405 CrossRef CAS.
- N. H. An, X. L. Yuan, B. Pan, Q. L. Li, S. Y. Li and W. X. Zhang, RSC Adv., 2014, 4, 38250–38257 RSC.
- S. Colussia, M. Boaroa, L. Rogatisa, A. Pappacenaa, C. Leitenburga, J. Llorcab and A. Trovarellia, Catal. Today, 2015, 253, 163–167 CrossRef.
- L. H. Nie, A. Y. Meng, J. G. Yu and M. Jaroniec, Sci. Rep., 2013, 3, 1–6 Search PubMed.
- Z. H. Xu, J. G. Yu and M. Jaroniec, Appl. Catal., B, 2015, 163, 306–312 CrossRef CAS.
- N. H. An, S. Y. Li, P. N. Duchesne, P. Wu, W. L. Zhang, J. F. Lee, S. Cheng, P. Zhang, M. J. Jia and W. X. Zhang, J. Phys. Chem. C, 2013, 117, 21254–21262 CAS.
- S. Y. Li, M. J. Jia, J. Gao, P. Wu, M. L. Yang, S. H. Huang, X. W. Dou, Y. Yang and W. X. Zhang, J. Phys. Chem. C, 2015, 119, 2483–2490 CAS.
- X. L. Tang, B. C. Zhang, Y. Li, Q. Xin and W. J. Shen, Chin. J. Catal., 2005, 26, 1–3 CAS.
- X. D. Wang, H. B. Yu, D. Y. Hua and S. H. Zhou, J. Phys. Chem. C, 2013, 117, 7294–7302 CAS.
- Y. Sakamoto, K. Higuchi, N. Takahashi, K. Yokota, H. Doi and M. Sugiura, Appl. Catal., B, 1999, 23, 159–167 CrossRef CAS.
- A. S. Ivanova, E. M. Slavinskaya, R. V. Gulyaev, V. I. Zaikovskii, O. A. Stonkus, I. G. Danilova, L. M. Plyasova, I. A. Polukhina and A. I. Boronin, Appl. Catal., B, 2010, 97, 57–71 CrossRef CAS.
- G. X. Chen, Y. Zhao, G. Fu, P. N. Duchesne, L. Gu, Y. P. Zheng, X. F. Weng, M. S. Chen, P. Zhang, C. W. Pao, J. F. Lee and N. F. Zheng, Science, 2014, 344, 495–499 CrossRef CAS PubMed.
- Q. Fu, Y. X. Yao, X. G. Guo, M. M. Wei, Y. X. Ning, H. Y. Liu, F. Yang, Z. Liu and X. H. Bao, Phys. Chem. Chem. Phys., 2013, 15, 14708–14714 RSC.
- J. Landon, E. Demeter, N. İnoğlu, C. Keturakis, I. E. Wachs, R. Vasić, A. I. Frenkel and J. R. Kitchin, ACS Catal., 2012, 2, 1793–1801 CrossRef CAS.
- N. H. An, P. Wu, S. Y. Li, M. J. Jia and W. X. Zhang, Appl. Surf. Sci., 2013, 285, 805–809 CrossRef CAS.
- L. Q. Liu, F. Zhou, L. G. Wang, X. J Qi, F. Shi and Y. Q. Deng, J. Catal., 2010, 274, 1–10 CrossRef CAS.
- Q. Fu, W. X. Li, Y. X. Yao, H. Y. Liu, H. Y. Su, D. Ma, X. K. Gu, L. M. Chen, Z. Wang, H. Zhang, B. Wang and X. H. Bao, Science, 2010, 328, 1141–1144 CrossRef PubMed.
- B. T. Qiao, A. Q. Wang, X. F. Yang, L. F. Allard, Z. Jiang, Y. T. Cui, J. Y. Liu, J. Li and T. Zhang, Nat. Chem., 2011, 3, 634–641 CrossRef CAS PubMed.
- B. B. Chen, X. B. Zhu, M. Crocker, Y. Wang and C. Shi, Appl. Catal., B, 2014, 154, 73–81 CrossRef.
- J. L. Wang, R. Yunus, J. G. Li, P. L. Li, P. Y. Zhang and J. Kim, Appl. Surf. Sci., 2015, 357, 787–794 CrossRef CAS.
- D. Z. Zhao, C. Shi, X. S. Li, A. M. Zhu and B. W. L. Jang, J. Hazard. Mater., 2012, 239–240, 362–369 CrossRef CAS PubMed.
- S. Y. Li, G. Liu, H. L. Lian, M. J. Jia, G. M. Zhao, D. Z. Jiang and W. X. Zhang, Catal. Commun., 2008, 9, 1045–1049 CrossRef CAS.
- A. Tomita, K. Shimizu, K. Kato, T. Akita and Y. Tai, J. Phys. Chem. C, 2013, 117, 1268–1277 CAS.
| This journal is © The Royal Society of Chemistry 2015 |


