Gold-catalyzed tandem cycloisomerization/Petasis–Ferrier rearrangement: a direct route to 3-alkoxyindanones from enynals and alcohols†
Abstract
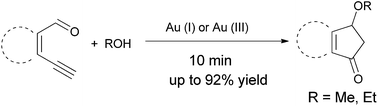
A method to prepare 3-alkoxyindanones efficiently by gold-catalyzed tandem cycloisomerization/Petasis–Ferrier rearrangement from ortho-ethynylarylaldehydes with alcohols is described. The reaction was accomplished in moderate to excellent yields under mild reaction conditions and also offers a convenient synthetic route to 3-alkoxycyclopentenones.

 Please wait while we load your content...
Please wait while we load your content...