Au@Cu7S4 yolk–shell nanoparticles as a 980 nm laser-driven photothermal agent with a heat conversion efficiency of 63%†
Jing Zhang‡
,
Guigao Liu‡,
Fang He*,
Lixia Chen and
Yuan Huang
Department of Materials Science and Engineering, Tianjin University, 92 Weijin Road, Nankai District, Tianjin, China. E-mail: fanghe@tju.edu.cn
First published on 9th October 2015
Abstract
We report the preparation of Au@Cu7S4 yolk–shell nanoparticles (NPs) via an inward replacement strategy based on the Kirkendall Effect. Due to the surface plasmon resonance (SPR) effect enhanced absorption in the near-infrared (NIR) region and the unique yolk–shell structure, the Au@Cu7S4 NPs exhibit excellent photothermal performance with a heat conversion efficiency up to 63% under 980 nm laser irradiation.
The photothermal effect of nanostructures as an emerging research spot has received increasing attention due to the promising applications in water splitting,1 organic degradation,2 CO2 reduction,3 Suzuki coupling reactions,4 and especially in cancer photothermal therapy.5–8 Generally, the photothermal effect can be described as the conversion of heat from photon energy, which involves the excitation of the surface plasmon band of nanostructures and the subsequent fast thermal relaxation of the exciton.9,10 Therefore, nanostructures with unique surface plasmon resonance (SPR) absorption properties have been widely explored as photothermal agents, such as Au based nanostructures (nano-rods,11 -cages,12,13 and -stars14), Pd-based nanostructures,15 carbon-based materials (carbon nanotubes16 and graphene17), copper sulfides (CuxS, 1 ≤ x ≤ 2)5,7,18–20 etc. Among them, due to their low cost, high stability, low cytotoxicity, and the intrinsic SPR induced NIR absorption properties, CuxS are becoming one class of most popular photothermal materials in recent years.5–7,20,21 Different CuxS (like flower-like CuS superstructures,20 hollow CuS nanoparticles (NPs),6 and Cu9S5 nanocrystals5) or CuxS based composites (like Fe3O4@ CuxS core–shell NPs,7 NaYbF4: 2%Er3+/20%Gd3+@SiO2–NH2–CuS,19 and Au–Cu9S5 hybrid NPs18) in various morphologies and structures were developed and investigated as photothermal agents. Despite these successes, the CuxS still suffer from relatively low photothermal conversion efficiency. The exploration of efficient CuxS photothermal agents remains great challenges.
Recently, Jiang et al. demonstrated that, as a result of coupling effect of SPR originating from the collective electron and hole oscillations, the combination of Au NPs with CuxS could significantly enhance SPR of CuxS and consequently improve the photothermal performance.18 On the other hand, we also noticed that the unique yolk–shell structure can give rise to the multiple reflections of light between cores and shells within the interior voids, beneficial for the full utilization of light energy, which has been widely applied for the designing efficient optical functional materials.22,23 Herein, inspired by these two phenomena, we carefully designed and synthesized Au@Cu7S4 yolk–shell NPs via an inward replacement strategy based on the Kirkendall Effect using Au@Cu2O core–shell NPs as templates. As expected, the materials showed a powerful photothermal performance with the heat conversion efficiency up to 63% under a 980 nm laser irradiation. It should be noted that, apart from being considered as a strategy for enhancing the photothermal properties of Cu7S4 NPs, the construction of such unique Au-contained yolk–shell structure might make the materials promising platforms as drug deliverers.23
As illustrated in Scheme 1, the preparation of Au@Cu7S4 yolk–shell nanoparticles is based on an inward replacement strategy using Au@Cu2O core–shell nanoparticles as templates, which typically involves two steps.24–26 Firstly, using Au nanoparticles as seeds and polyvinylpyrrolidone (PVP) as a structural directing agent, a polycrystalline Cu2O layer was formed and coated outside the Au cores to get an Au@Cu2O core–shell structure.27 Secondly, under air atmosphere, Cu2O shells would be converted into Cu7S4 shells immediately when suspended in the Na2S solution because of the much smaller solubility product constant of Cu7S4 (Ksp ≈ 10−48) than that of Cu2O (eqn (1)).25
| 14Cu2O + 16S2− + O2 + 16H2O → 4Cu7S4 + 32OH− | (1) |
Owing to the Kirkendall Effect induced net directional flow of materials at the template/reactant interface, the voids would be appeared in Cu7S4 shells accompanied by the increasing of particle diameter, consequently resulting in the formation of Au@Cu7S4 yolk–shell structures.24,25
Au NPs were prepared by a standard citrate reduction procedure28 and further observed by transmission electron microscopy (TEM). As shown in Fig. 1a and S1,† they exhibit uniform spherical morphology with high crystallinity and possess an average diameter of 20.3 ± 2.6 nm. Fig. 1b is the TEM image of Au@Cu2O nanoparticles. It is apparent that they show typical core–shell structures and that each of them only contains one individual Au NP core. The high-resolution TEM (HRTEM) image in Fig. 1c and the Selected Area Electron Diffraction (SAED) pattern in Fig. S2† confirm the polycrystalline characteristics of the Cu2O shells, which is well consistent with the previous report.27 Specifically, the lattice spacings of 0.243 and 0.300 nm corresponds to the (111) and (110) planes of Cu2O, respectively.29 The morphology and interior structure of Au@Cu7S4 NPs are showed in Fig. 1d and e. Compared with Au@Cu2O, obvious voids were formed in Au@Cu7S4 NPs, transferring the structure from core–shell to yolk–shell. This is believed to be the result of Kirkendall Effect.24 Meanwhile, we also notice that many nanoplates were growing from the polycrystalline Cu7S4 shell. This result suggested that the structure reconstruction of Cu7S4 occurred during the replacement reaction. The particle size distribution histograms for Au@Cu2O and Au@Cu7S4 are drawn in Fig. 1f and their average particle radii are determined from the mean value of Gaussian distribution to be 80.3 and 88.5 nm, respectively. This observed increase in diameters further confirms the formation of hollow interior inside the Cu7S4 shells. In addition, it should be noted that, apart from endowing such composite materials many attractive properties, like plasmonic effect, Au NPs also play critical roles in mediating the growth of Cu2O in nanoscales. The pure Cu2O particles were prepared in the absence of Au NPs as control experiments. As expected, they show a much larger particle size of around 200 nm (Fig. S3†). The similar conclusion was also obtained by others, who demonstrated that the diameter of Au@Cu2O NPs could be tailored by controlling the adding amount of Au particle seeds.27
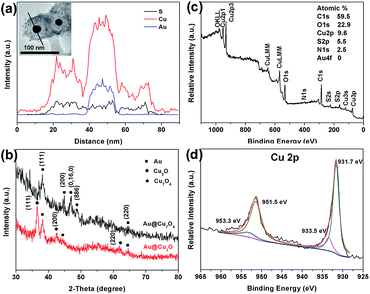
Dispersed X-ray Spectroscopy (EDX) spectroscopy was further performed on Au@Cu7S4 NPs to reveal the formation of yolk–shell structure. Fig. S4† shows an integrated EDX spectrum of Au@Cu7S4 NPs, in which the signals belonging to Au, Cu, and S are detected, indicating the transfer from Au@Cu2O to Au@Cu7S4. The EDX line-scanning across an individual Au@Cu7S4 NP is displayed in Fig. 2a, which clearly suggests that Au is strictly confined in the central core area, while S signal is distributed over the whole cross-section of the particle with a slight higher intensity at the shell. It is noteworthy that the Cu line scans contain the excited Cu X-rays from both the sample and the Cu grid. The significant increase in the signal of Cu at the Au core region is largely due to the contribution from the Cu grid, which is greatly enhanced when strong electron scattering from the Au core occurred during the line-scan process.27
To provide more evidences to support the formation of Au@Cu7S4 from Au@Cu2O, the X-ray-diffraction (XRD) analysis was also conducted. Before the measurements, the sample suspensions were dip-coated on a silicon substrate and then air-dried to obtain the films, which were used for the characterization. As shown in Fig. 2b, the XRD diffraction peaks from cubic-phase Au are detected in both Au@Cu2O and Au@Cu7S4 samples, indicating the existence of Au. Besides these, the peaks from Cu2O (PDF file no. 05-0667) and Cu7S4 (PDF file no. 23-0598) are also recognized, respectively, for the Au@Cu2O and Au@Cu7S4, though a poor quality in peak signals is presented.
X-ray photoelectron spectroscopy (XPS) technique was further employed to confirm the chemical states of Cu in Au@Cu7S4 yolk–shell structures. Fig. 2c shows the survey spectrum of Au@Cu7S4 NPs, from which the peaks of Cu, and S elements with atomic concentrations of 9.6 and 5.5%, respectively, are observed. And the Cu/S molar ratio of the sample was calculated to be 1.745, close to the theoretic value of idealized Cu7S4. Additionally, a trace amount of O and N elements are also found, which can be attributed to the adsorbed PVP molecules. Since XPS is a surface analysis technique with investigation depth of 2–5 nm and the Au cores are coated by Cu7S4, the signal of Au element is not detected.30 Additionally, in Fig. 2d, the highly resolved XPS spectrum of Cu 2p is fitted to four peaks. The main peaks located at 937.1 eV and 951.5 eV can be attributed to the Cu 2p3/2 and Cu 2p1/2 of Cu+, respectively.31,32 The shake-up satellite peaks at 933.5 eV and 953.3 eV are evidence for the presence of Cu2+.31,32 By integrating the peak area, the molar ratio of Cu+/Cu2+ is estimated to be 6.0, well agreement with the value in Cu7S4. Based on these results, it can be concluded that the Cu7S4 was formed by treating Cu2O in Na2S solutions.
The optical properties of the aqueous dispersion containing Au@Cu7S4 NPs were examined by UV-vis-NIR spectroscopy, and for a comparison, the Au and Au@Cu2O NP suspensions were also investigated. In Fig. 3a, the Au NPs shows a typical plasmonic absorption in visible-light range with the peak centered at 520 nm. After coated by the Cu2O shells, the Au absorption is clearly red-shifted to 566 nm, which can be ascribed to the high refractive index of Cu7S4 surrounding the Au cores.33 Interestingly, this absorption is shifted to around 520 nm again in Au@Cu7S4 NP suspensions. This result provides an additional evidence supporting the formation of voids between the Au cores and Cu7S4 shells. More importantly, Au@Cu7S4 NPs show an enhanced absorption with the increase of wavelength in the NIR region (700–1400 nm). This NIR absorbance of the Au@Cu7S4 NPs is mainly attributed to the localized surface plasmon resonances (SPR) of Cu7S4 resulted from Cu deficiencies in the structure.5
The strong SPR absorption of Au@Cu7S4 yolk–shell NPs in NIR region motivates us to further investigate their photothermal properties using a 980 nm laser at the plasmon band, which can gain an insight into their potential applications for photothermal cancer therapy. In Fig. 3b, we show the temperature elevation of pure water (as a reference) and Au@Cu7S4 aqueous dispersions with different concentrations under the irradiation of 980 nm laser. At laser powder density of 0.51 W cm−2, with the NP concentration varied from 0.06 to 2.00 g L−1, the temperature elevation of the aqueous dispersions increases from 6.0 to 11.1 °C after 7 min laser irradiation, while the pure water exhibits little heating at the same conditions. These results indicate that the Au@Cu7S4 NPs can rapidly and efficiently convert the NIR energy into thermal energy. The final temperature changes at the end of the laser irradiation period as a function of nanoparticle concentrations are plotted in the inset of Fig. 3b. It is clear that the temperature change elevation profile rises up dramatically with the concentration of Au@Cu7S4 NPs increasing to 0.25 g L−1 and then flattens out upon the concentration further increasing to 2.0 g L−1. This phenomenon might be attributed to a relatively fast heat loss at high temperature and the logarithmic absorbance dependence of Au@Cu7S4 NPs on the fraction of incident radiation.5,18
A modified method reported by Roper et al. was performed to calculate the photothermal conversion efficiency of the Au@Cu7S4 yolk–shell NPs.34 As shown in Fig. 4a, the Au@Cu7S4 aqueous dispersions with a concentration of 2.0 g L−1 was kept under a continuous illumination by the 980 nm laser until reaching a steady state temperature. Subsequently, the laser was removed and the dispersions was allowed to cool down naturally. By fitting the measured cooling curve (Fig. 4b), the rate of heat dissipation from the system to the environment could be obtained. According to Roper's report, the photothermal conversion efficiency, η, was calculated using the following equation (eqn (2), detailed in the ESI†):34
 | (2) |
Conclusions
In summary, we have successfully demonstrated the yolk–shell structured Au@Cu7S4 NPs through a wet chemistry approach, which involves the growth of polycrystalline Cu2O shells outside Au NP cores and subsequent inward replacement of the Cu2O in a S2− contained solution leading to the formation of hollow Cu7S4 shells based on the Kirkendall Effect. Due to localized surface plasmon resonance (SPR) effect and the unique yolk–shell structure, the as-prepared Au@Cu7S4 yolk–shell NPs exhibit an enhanced absorption with the increase of wavelength in near-infrared (NIR) region. Under the irradiation of a 980 nm laser, the materials show a high photothermal conversion efficiency of 63%, which results in the increase of the temperature of their aqueous dispersions by 11.1 °C in 7 min. This feature makes Au@Cu7S4 NPs potential candidates for photothermal therapy applications. Furthermore, the designed Au NP-contained yolk–shell structure may also hold great promise for drug delivery.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No: 51372169).Notes and references
- W. C. Chueh, C. Falter, M. Abbott, D. Scipio, P. Furler, S. M. Haile and A. Steinfeld, Science, 2010, 330, 1797–1801 CrossRef CAS PubMed.
- L. Liu, S. Ouyang and J. Ye, Angew. Chem., Int. Ed., 2013, 52, 6689–6693 CrossRef CAS PubMed.
- X. Meng, T. Wang, L. Liu, S. Ouyang, P. Li, H. Hu, T. Kako, H. Iwai, A. Tanaka and J. Ye, Angew. Chem., Int. Ed., 2014, 53, 11478–11482 CrossRef CAS PubMed.
- F. Wang, C. Li, H. Chen, R. Jiang, L.-D. Sun, Q. Li, J. Wang, J. C. Yu and C.-H. Yan, J. Am. Chem. Soc., 2013, 135, 5588–5601 CrossRef CAS PubMed.
- Q. Tian, F. Jiang, R. Zou, Q. Liu, Z. Chen, M. Zhu, S. Yang, J. Wang, J. Wang and J. Hu, ACS Nano, 2011, 5, 9761–9771 CrossRef CAS PubMed.
- K. Dong, Z. Liu, Z. Li, J. Ren and X. Qu, Adv. Mater., 2013, 25, 4452–4458 CrossRef CAS PubMed.
- Q. Tian, J. Hu, Y. Zhu, R. Zou, Z. Chen, S. Yang, R. Li, Q. Su, Y. Han and X. Liu, J. Am. Chem. Soc., 2013, 135, 8571–8577 CrossRef CAS PubMed.
- B. Li, Q. Wang, R. Zou, X. Liu, K. Xu, W. Li and J. Hu, Nanoscale, 2014, 6, 3274–3282 RSC.
- S. Linic, P. Christopher and D. B. Ingram, Nat. Mater., 2011, 10, 911–921 CrossRef CAS PubMed.
- J. Qiu and W. D. Wei, J. Phys. Chem. C, 2014, 118, 20735–20749 CAS.
- H. Chen, L. Shao, T. Ming, Z. Sun, C. Zhao, B. Yang and J. Wang, Small, 2010, 6, 2272–2280 CrossRef CAS PubMed.
- L. Au, D. Zheng, F. Zhou, Z.-Y. Li, X. Li and Y. Xia, ACS Nano, 2008, 2, 1645–1652 CrossRef CAS PubMed.
- J. Chen, D. Wang, J. Xi, L. Au, A. Siekkinen, A. Warsen, Z.-Y. Li, H. Zhang, Y. Xia and X. Li, Nano Lett., 2007, 7, 1318–1322 CrossRef CAS PubMed.
- H. Yuan, A. M. Fales and T. Vo-Dinh, J. Am. Chem. Soc., 2012, 134, 11358–11361 CrossRef CAS PubMed.
- X. Huang, S. Tang, X. Mu, Y. Dai, G. Chen, Z. Zhou, F. Ruan, Z. Yang and N. Zheng, Nat. Nanotechnol., 2011, 6, 28–32 CrossRef CAS PubMed.
- N. W. S. Kam, M. O'Connell, J. A. Wisdom and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11600–11605 CrossRef CAS PubMed.
- L. Feng, X. Yang, X. Shi, X. Tan, R. Peng, J. Wang and Z. Liu, Small, 2013, 9, 1989–1997 CrossRef CAS PubMed.
- X. Ding, C. H. Liow, M. Zhang, R. Huang, C. Li, H. Shen, M. Liu, Y. Zou, N. Gao, Z. Zhang, Y. Li, Q. Wang, S. Li and J. Jiang, J. Am. Chem. Soc., 2014, 136, 15684–15693 CrossRef CAS PubMed.
- Q. Xiao, X. Zheng, W. Bu, W. Ge, S. Zhang, F. Chen, H. Xing, Q. Ren, W. Fan, K. Zhao, Y. Hua and J. Shi, J. Am. Chem. Soc., 2013, 135, 13041–13048 CrossRef CAS PubMed.
- Q. Tian, M. Tang, Y. Sun, R. Zou, Z. Chen, M. Zhu, S. Yang, J. Wang, J. Wang and J. Hu, Adv. Mater., 2011, 23, 3542–3547 CrossRef CAS PubMed.
- Y. Zhao, H. Pan, Y. Lou, X. Qiu, J. Zhu and C. Burda, J. Am. Chem. Soc., 2009, 131, 4253–4261 CrossRef CAS PubMed.
- H. Li, Z. Bian, J. Zhu, D. Zhang, G. Li, Y. Huo, H. Li and Y. Lu, J. Am. Chem. Soc., 2007, 129, 8406–8407 CrossRef CAS PubMed.
- J. Liu, S. Z. Qiao, J. S. Chen, X. W. Lou, X. Xing and G. Q. Lu, Chem. Commun., 2011, 47, 12578–12591 RSC.
- H. Cao, X. Qian, C. Wang, X. Ma, J. Yin and Z. Zhu, J. Am. Chem. Soc., 2005, 127, 16024–16025 CrossRef CAS PubMed.
- S. Jiao, L. Xu, K. Jiang and D. Xu, Adv. Mater., 2006, 18, 1174–1177 CrossRef CAS PubMed.
- C.-H. Kuo, Y.-T. Chu, Y.-F. Song and M. H. Huang, Adv. Funct. Mater., 2011, 21, 792–797 CrossRef CAS PubMed.
- L. Zhang, D. A. Blom and H. Wang, Chem. Mater., 2011, 23, 4587–4598 CrossRef CAS.
- X. Ji, X. Song, J. Li, Y. Bai, W. Yang and X. Peng, J. Am. Chem. Soc., 2007, 129, 13939–13948 CrossRef CAS PubMed.
- C.-H. Kuo, T.-E. Hua and M. H. Huang, J. Am. Chem. Soc., 2009, 131, 17871–17878 CrossRef CAS PubMed.
- G. Liu, F. He, J. Zhang, L. Li, F. Li, L. Chen and Y. Huang, Appl. Catal., B, 2014, 150–151, 515–522 CrossRef CAS PubMed.
- R. Cai, J. Chen, J. Zhu, C. Xu, W. Zhang, C. Zhang, W. Shi, H. Tan, D. Yang, H. H. Hng, T. M. Lim and Q. Yan, J. Phys. Chem. C, 2012, 116, 12468–12474 CAS.
- X. Jiang, Y. Xie, J. Lu, W. He, L. Zhu and Y. Qian, J. Mater. Chem., 2000, 10, 2193–2196 RSC.
- P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Nano Today, 2007, 2, 18–29 CrossRef.
- D. K. Roper, W. Ahn and M. Hoepfner, J. Phys. Chem. C, 2007, 111, 3636–3641 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and additional experimental results. See DOI: 10.1039/c5ra19055j |
| ‡ Both J. Zhang and G. Liu have contributed equally towards the work. |
| This journal is © The Royal Society of Chemistry 2015 |