Enhancement of electron-donating character in alkylated monopyrrolo-tetrathiafulvalene derivatives†
Kai R. Korschinga,
Hannes Schäfera,
Josefine Schönborna,
Arunpatcha Nimthong-Roldánb,
Matthias Zellerb and
Vladimir A. Azov*a
aUniversity of Bremen, Department of Chemistry, Leobener Str. NW 2C, D-28359 Bremen, Germany. E-mail: vazov@uni-bremen.de; vlazov@gmail.com
bOne University Plaza, Youngstown State University, Department of Chemistry, Youngstown, OH 44555-3663, USA
First published on 23rd September 2015
Abstract
Herein we report the first synthesis of a monopyrrolo-tetrathiafulvalene (MPTTF) with two alkyl substituents, performed using a triphenylphosphine-mediated coupling reaction. An Ullmann-type N-arylation reaction was used to prepare a calixarene-based bis-MPTTF receptor, as well as an arylated mono-MPTTF derivative. Complexation studies with electron-deficient compounds showed strongly enhanced affinity of the alkyl-substituted MPTTF derivatives in comparison with the non-substituted MPTTF analogues.
Tetrathiafulvalene1 (TTF) and its derivatives are redox active heterocyclic compounds that first found use in the field of organic electronics2 because of their ability to form various types of electrically conductive solid phases. Due to the fact that the strong electron donating properties of TTFs can be turned off by reversible oxidation,3 TTF subunits have been exploited in a number of supramolecular systems with redox control, such as organogels,4 molecular sensors,5 functional materials6 and interlocked supramolecular systems, e.g. catenanes and rotaxanes.7 In the latter TTF-containing interlocked molecular architectures,7 a single TTF moiety was reversibly bound inside a cyclobis(paraquat-p-phenylene) macrocyle, being sandwiched between two electron-deficient 4,4′-bipyridyl moieties.
Examples of the reversed design of molecular receptors, which comprise several TTF groups serving as a molecular recognition centre for single electron deficient guests, are much less common. TTF-calix[4]pyrrole receptors developed in a collaboration between the Sessler and Jeppensen groups contain four monopyrrolo-tetrathiafulvalene8 (MPTTF) units, which form a molecular recognition center.9 The possibility of switching the calix[4]pyrrole cores between the alternate and cone conformations allowed the employment of these electron-rich receptors for the binding of two different substrate classes: either planar electron-deficient aromatic molecules, or spacious spherical fullerenes. These receptors were employed for investigation of fundamental processes, such as electron transfer in supramolecular systems.10 Additionally, this class of receptors was used to create supramolecular polymers11 and molecular sensors for nitro aromatics,12 pointing out a path towards practical applications. Glycoluril-based bis-TTF molecular architectures designed in the groups of Chiu13 and Hudhomme14 constitute two additional rare examples of molecular tweezers with TTF arms.
In our previous work, we employed MPTTF derivatives for the synthesis of molecular receptors with a molecular tweezers architecture. The basal subunit of such a receptor consists of a calix[4]arene15 or a 1,3,5-substituted 2,4,6-triethylbenzene scaffold16 to which two or three TTF units are attached via the apex N-atoms of the pyrrolo groups. The MPTTF units make up the tongues of the tweezers, between which an electron-deficient guest molecule can be bound. Bis-MPTTF-calix[4]arene molecular tweezers displayed a high binding affinity towards electron-deficient substances. As an added benefit, these molecular receptors are rich in sites that might allow for further synthetic modifications.
In this communication we report the first synthesis and characterization of bis-methyl-substituted monopyrrolo-tetrathiafulvalene 1, its application in Cu(I)-catalyzed N-arylation reactions, and investigations into the binding properties of its mono- and bis-derivatives with electron-deficient substrates. Additionally, we report the formation of a previously unknown side product in the phosphite-mediated ketone–thione cross-coupling reaction.
The synthesis of bis-methyl substituted MPTTF derivative 1 is shown in Scheme 1. First, N-tosylated MPTTF derivative 2 was prepared using a standard phosphite-mediated cross-coupling reaction between dithiole–ketone 3 and dithiole–thione 4 by heating in neat triethylphosphite. Analysis of the reaction mixture revealed the presence of a second colourless product 5 in addition to N-tosylated MPTTF derivative 2, with a very similar Rf value, identical molecular mass and almost indistinguishable 1H and 13C NMR spectra (ESI†).
Differences in solubilities allowed us to separate 2 and 5. X-ray structure determination† revealed that compound 5 has a tricyclic backbone with two annealed six-membered dithiine type rings (Fig. 1). Both rings are folded along the S–S vectors, rendering the chair-like conformation to the annealed ring system. Structurally similar bicyclic 1,4,5,8-tetrathianaphthalene had been reported to rearrange into a tetrathiafulvalene after treatment with a large excess of a strong base, such as LDA or tBuOK, in THF,17 but no examples of the reverse reaction have been reported to date. Heating of 5 for prolonged periods of time (up to 4 h) in hot P(OEt)3 did not lead to its rearrangement into 2, indicating that 5 forms via an unreported side branch of the commonly accepted mechanism of tetrathiafulvalene formation.18
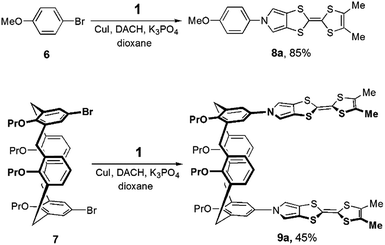
Treatment of the tosylated derivative 2 with sodium methanoate solution afforded the target compound 1 as a yellow crystalline powder. The reactivity of MPTTF 1 towards Cu(I) catalyzed N-arylation was tested by reaction with bromoanisole 6 and with bis-brominated calix[4]arene derivative 7 (Scheme 2). Both reactions afforded the desired mono- and bis-MPTTF derivatives 8a and 9a, respectively, as bright yellow crystalline powders stable under ambient conditions. Compound 9a features a molecular tweezers architecture that can serve as a potential host for electron-deficient molecular guests.
The crystal structure of 8a† unambiguously confirmed the constitution of the dimethyl-substituted MPTTF scaffold (Fig. 2). In the crystal, the MPTTF moiety of 8 features an almost planar arrangement, with a maximum deviation of fitted atoms from the least-square plane, defined by heavy atoms of the MPTTF backbone, of 0.139 Å. The molecule as a whole is twisted, with an angle of 36.18° between the two least-square planes of the pyrrole and benzene rings, defined by their heavy atoms.
 | ||
| Fig. 2 X-ray structure of arylated MPTTF derivative 8a. Thermal ellipsoids are shown at the 50% probability level. | ||
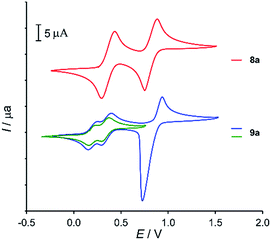
New MPTTF derivatives 1, 8a, and 9a were investigated using cyclic voltammetry in CH2Cl2 (Table 1). Compound 1 displays only one quasi-reversible oxidation at a potential of 0.18 V, whereas the second oxidation wave is irreversible. Both N-arylated derivatives 8a and 9a show the common tetrathiafulvalene behaviour with two TTF-centred oxidation potentials, the first leading to formation of the radical-cation, and at the second potential the dication is formed (Fig. 3). Bis-MPTTF derivative 9a displays a prominent splitting of the first oxidation wave, which had been occasionally observed for tetrathiafulvalene derivatives with two or more spatially proximal TTF groups.19 This effect was explained by the formation of mixed-valence tetrathiafulvalene dimers (TTF2)+˙, stabilized by intramolecular charge-transfer interactions between TTF and TTF+˙ moieties.20 The splitting observed in the case of 9a is much stronger when compared to cyclic voltammograms of similar calixarene-based bis-MPTTF molecular tweezers with less electron-donating substituents (SPr or H-atoms) at the TTF group.15
| Compound | Eox1.11/2 (V) | Eox1.21/2 (V) | Eox21/2 (V) |
|---|---|---|---|
| a Data were obtained using a one-compartment cell in CH2Cl2/0.1 M Bu4NClO4, Pt as the working and counter electrodes and a non-aqueous Ag/Ag+ reference electrode; scan rate 100 mV s−1. Values given at room temperature vs. SCE; the Fc/Fc+ couple (0.480 V vs. SCE) was used as a reference.21 | |||
| 1 | 0.18 | — | — |
| 8a | 0.41 | — | 0.84 |
| 9a | 0.17 | 0.33 | 0.80 |
Binding studies† were performed to evaluate the electron-donation properties of the alkyl-substituted MPTTF derivatives 8a and 9a in comparison with their non-substituted counterparts15 8b and 9b (Fig. 4). Tetracyanoquino-dimethane 10 (TCNQ) was used as the guest for molecular tweezers 9a,b, whereas cyclobis(paraquat-p-phenylene) macrocycle 11 (ref. 22) (CBPQT4+) was used as molecular host for MPTTFs 8a,b (Fig. 5). Pairwise mixing of host–guest solutions leads to formation of deeply-coloured charge-transfer complexes, easily visible to the naked eye and showing intense charge-transfer bands in their UV/vis spectra above 600 nm. TCNQ gives an intense green colour with molecular tweezers 9a,b, whereas mixing of compounds 8a,b with CBPQT4+ leads to formation of green-yellowish-coloured solutions.
 | ||
| Fig. 4 Structures of the donor-type (8a,b and 9a,b) and acceptor-type (10 and 11) molecular guests/hosts. | ||
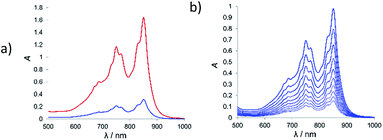
Neither the MPTTF derivatives, nor any of the acceptors show absorption bands in the wavelength range of 500 to 1100 nm, which allowed determination of binding constants using UV/vis spectroscopy in dichloromethane (for 9a,b) or Me2CO (for 8a,b). Initial qualitative experiments gave evidence of a very high binding affinity for the 9a/TCNQ host–guest pair, prompting us to use the dilution method,23 which is well suited for the determination of very high binding constants.
Molecular tweezers 9a exhibits a remarkably high affinity towards TCNQ with a binding constant of Ka = 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 and a very strong extinction coefficient for the complex ε = 9200 M−1 cm−1 (Table 2). Receptor 9b shows a lower binding constant of 90
000 M−1 and a very strong extinction coefficient for the complex ε = 9200 M−1 cm−1 (Table 2). Receptor 9b shows a lower binding constant of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 and an almost five-fold decrease of the complex extinction coefficient. Comparison of MPTTF derivatives 8a and 8b in binding experiments with CBPQT4+ also showed increased binding affinity of the methyl-substituted MPTTF 8a in comparison with its non-substituted counterpart 8b (Table 2).
000 M−1 and an almost five-fold decrease of the complex extinction coefficient. Comparison of MPTTF derivatives 8a and 8b in binding experiments with CBPQT4+ also showed increased binding affinity of the methyl-substituted MPTTF 8a in comparison with its non-substituted counterpart 8b (Table 2).
| Compound | Ka, M−1 (ε, M−1 cm−1) with 10b | Ka, M−1 (ε, M−1 cm−1) with 11c |
|---|---|---|
| a Data were obtained in CH2Cl2 (9a,b) or Me2CO (8a,b) solns using UV/vis dilution method at room temperature.b Absorption maximum for CT complexes of 10 with receptors 9a,b lies at 850 nm.c Absorption maxima for CT complexes of 11 with receptors 8a,b lie at 876 nm and 854 nm, respectively. | ||
| 9a | 2.5 × 105 (9200) | — |
| 9b | 9.5 × 104 (1900) | — |
| 8a | — | 6200 (2600) |
| 8b | — | 4200 (1900) |
The differences in binding affinity between the non-substituted and methylated MPTTF derivatives can be attributed to the electron-donating effect of the Me-groups, affording an increase in electron density on the TTF units. Combined with good chemical stability, this increase in donor ability renders Me-substituted MPTTF a highly promising building block for the construction of electron-rich redox-active molecular architectures.
In conclusion, we have performed the first synthesis of novel alkyl-substituted monopyrrolotetrathiafulvalene 1 and successfully employed it in a copper-catalyzed arylation reaction. New MPTTF derivatives displayed good chemical stability combined with low oxidation potentials and exceptional electron-donating properties. Calix[4]arene-based molecular tweezers 9a shows an extremely high affinity for TCNQ, forming stable charge-transfer complexes with very high extinction coefficients. Such molecular architecture should serve as a headstone for the development of efficient hosts for other classes of electron-deficient guests. Formation of an unexpected byproduct 5 in the phosphite-mediated coupling between 3 and 4 requires a deeper look into the mechanism of this reaction.
Acknowledgements
The X-ray diffractometer was funded by NSF Grant 0087210, Ohio Board of Regents Grant CAP-491, and by Youngstown State University. We are grateful to Dr T. Dülcks, Ms D. Kemken (MS), Dr Uli Papke (HR-MS, TU Braunschweig), and Dr J. Warneke (NMR) for their help with the characterization of the new compounds.Notes and references
- TTF Chemistry. Fundamentals and Applications of Tetrathiafulvalene, ed. J. Yamada and T. Sugimoto, Springer, Heidelberg, 2004 Search PubMed.
- M. Bendikov, F. Wudl and D. F. Perepichka, Chem. Rev., 2004, 104, 4891–4945 CrossRef CAS PubMed.
- D. Canevet, M. Sallé, G. Zhang, D. Zhang and D. Zhu, Chem. Commun., 2009, 2245–2269 RSC.
- (a) J. Puigmart-Luis, V. Laukhin, A. P. del Pino, J. Vidal-Gancedo, C. Rovira, E. Laukhina and D. B. Amabilino, Angew. Chem., Int. Ed., 2007, 46, 238–241 CrossRef PubMed; (b) T. Kitahara, M. Shirakawa, S. Kawano, U. Beginn, N. Fujita and S. Shinkai, J. Am. Chem. Soc., 2005, 127, 14980–14981 CrossRef CAS PubMed.
- M. B. Nielsen, C. Lomholt and J. Becher, Chem. Soc. Rev., 2000, 29, 153–164 RSC.
- M. R. Bryce, J. Mater. Chem., 2000, 10, 589–598 RSC.
- N. N. P. Moonen, A. H. Flood, J. M. Fernández and J. F. Stoddart, Top. Curr. Chem., 2005, 262, 99–132 CrossRef CAS.
- (a) J. O. Jeppesen, K. Takimiya, F. Jensen, T. Brimert, K. Nielsen, N. Thorup and J. Becher, J. Org. Chem., 2000, 65, 5794–5805 CrossRef CAS PubMed; (b) J. O. Jeppesen and J. Becher, Eur. J. Org. Chem., 2003, 3245–3266 CrossRef CAS PubMed.
- (a) K. A. Nielsen, W.-S. Cho, J. Lyskawa, E. Levillain, V. M. Lynch, J. L. Sessler and J. O. Jeppesen, J. Am. Chem. Soc., 2006, 128, 2444–2451 CrossRef CAS PubMed; (b) K. A. Nielsen, G. H. Sarova, L. Martín-Gomis, F. Fernández-Lázaro, P. C. Stein, L. Sanguinet, E. Levillain, J. L. Sessler, D. M. Guldi, Á. Sastre-Santos and J. O. Jeppesen, J. Am. Chem. Soc., 2008, 130, 460–462 CrossRef CAS PubMed; (c) K. A. Nielsen, L. Martín-Gomis, G. H. Sarova, L. Sanguinet, D. E. Gross, F. Fernández-Lázaro, P. C. Stein, E. Levillain, J. L. Sessler, D. M. Guldi, Á. Sastre-Santos and J. O. Jeppesen, Tetrahedron, 2008, 64, 8449–8463 CrossRef CAS PubMed.
- (a) J. S. Park, E. Karnas, K. Ohkubo, P. Chen, K. M. Kadish, S. Fukuzumi, C. W. Bielawski, T. W. Hudnall, V. M. Lynch and J. L. Sessler, Science, 2010, 329, 1324–1327 CrossRef CAS PubMed; (b) C. M. Davis, Y. Kawashima, K. Ohkubo, J. M. Lim, D. Kim, S. Fukuzumi and J. L. Sessler, J. Phys. Chem. C, 2014, 118, 13503–13513 CrossRef CAS.
- J. S. Park, K. Y. Yoon, D. S. Kim, V. M. Lynch, C. W. Bielawski, K. P. Johnston and J. L. Sessler, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 20913–20917 CrossRef CAS PubMed.
- (a) J. S. Park, F. Le Derf, C. M. Bejger, V. M. Lynch, J. L. Sessler, K. A. Nielsen, C. Johnsen and J. O. Jeppesen, Chem.–Eur. J., 2010, 16, 848–854 CrossRef CAS PubMed; (b) K. A. Nielsen, S. Steffen Bähring and J. O. Jeppesen, Chem.–Eur. J., 2011, 17, 11001–11007 CAS.
- (a) K.-W. Cheng, C.-C. Lai, P.-T. Chianga and S.-H. Chiu, Chem. Commun., 2006, 2854–2856 RSC; (b) P.-T. Chiang, P.-N. Cheng, C.-F. Lin, Y.-H. Liu, C.-C. Lai, S.-M. Peng and S.-H. Chiu, Chem.–Eur. J., 2006, 12, 865–876 CrossRef CAS PubMed.
- (a) Y. Cotelle, M. Allain, S. Legoupy and P. Hudhomme, Org. Lett., 2014, 16, 2590–2593 CrossRef CAS PubMed; (b) M. Hardouin-Lerouge, Y. Cotelle, S. Legoupy and P. Hudhomme, New J. Chem., 2014, 38, 5341–5348 RSC.
- M. H. Düker, H. Schäfer, M. Zeller and V. A. Azov, J. Org. Chem., 2013, 78, 4905–4912 CrossRef PubMed.
- M.-L. L. Watat, T. Dülcks, D. Kemken and V. A. Azov, Tetrahedron Lett., 2014, 55, 741–744 CrossRef CAS PubMed.
- (a) S. Nakatsuji, Y. Amano, H. Kawamura and H. Anzai, J. Chem. Soc., Chem. Commun., 1994, 841–842 RSC; (b) R. L. Meline and R. L. Elsenbaumer, J. Chem. Soc., Perkin Trans. 1, 1998, 2467–2469 RSC.
- R. D. McCullough, M. A. Petruska and J. A. Belot, Tetrahedron, 1999, 55, 9979–9998 CrossRef CAS.
- (a) J. Lyskawa, M. Sallé, J.-Y. Balandier, F. Le Derf, E. Levillain, M. Allain, P. Viel and S. Palacin, Chem. Commun., 2006, 2233–2235 RSC; (b) P. Blanchard, N. Svenstrup and J. Becher, Chem. Commun., 1996, 615–616 RSC; (c) J. M. Spruell, A. Coskun, D. C. Friedman, R. S. Forgan, A. A. Sarjeant, A. Trabolsi, A. C. Fahrenbach, G. Barin, W. F. Paxton, S. K. Dey, M. A. Olson, D. Benítez, E. Tkatchouk, M. T. Colvin, R. Carmielli, S. T. Caldwell, G. M. Rosair, S. G. Hewage, F. Duclairoir, J. L. Seymour, A. M. Z. Slawin, W. A. Goddard III, M. R. Wasielewski, G. Cooke and J. F. Stoddart, Nat. Chem., 2010, 2, 870–879 CrossRef CAS PubMed; (d) M. Skibiński, R. Gómez, E. Lork and V. A. Azov, Tetrahedron, 2009, 65, 10348–10354 CrossRef PubMed.
- (a) S. V. Rosokha and J. K. Kochi, J. Am. Chem. Soc., 2007, 129, 828–838 CrossRef CAS PubMed; (b) M. Yoshizawa, K. Kumazawa and M. Fujita, J. Am. Chem. Soc., 2005, 127, 13456–13457 CrossRef CAS PubMed.
- (a) G. Gritzner and J. Kuta, Pure Appl. Chem., 1984, 56, 461–466 CrossRef; (b) N. G. Connely and W. E. Geiger, Chem. Rev., 1996, 96, 877–910 CrossRef PubMed.
- B. Odell, M. V. Reddington, A. M. Z. Slawin, N. Spencer, J. F. Stoddart and D. J. Williams, Angew. Chem., Int. Ed. Engl., 1988, 27, 1547–1550 CrossRef PubMed.
- M. B. Nielsen, J. O. Jeppesen, J. Lau, C. Lomholt, D. Damgaard, J. P. Jacobsen, J. Becher and J. F. Stoddart, J. Org. Chem., 2001, 66, 3559–3563 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, 1H NMR, 13C NMR, UV/vis spectra, details on electrochemical characterization and determination of the binding constants, X-ray crystal data of 5 (CCDC 1415145) and 8b (CCDC 1415146). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra19012f |
| This journal is © The Royal Society of Chemistry 2015 |