A molecular dynamics study on thermal and mechanical properties of graphene–paraffin nanocomposites
Abstract
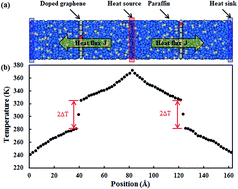
Owing to the superior thermal conductivity of graphene, nanocomposites with graphene fillers dispersed in a polymer matrix become promising in thermal management applications, e.g. serving as thermal interface materials (TIMs) in high power microelectronic devices. However, the thermal conductivity of graphene-based nanocomposites is constrained by the high interfacial thermal resistance between the graphene fillers and polymer matrix. This research focuses on changing graphene–paraffin interfacial thermal transport by employing various treatment methods. Using molecular dynamics (MD) simulations, the effectiveness of hydrogenation, defecting and doping on reducing the graphene–paraffin interfacial thermal resistance is closely investigated. We found that the interfacial thermal resistance can be considerably reduced by the hydrogenation of graphene, while it is insensitive to defecting and doping. From the simulation results of the graphene–paraffin nanocomposites under tensile loading, a lower Young’s modulus and lower tensile strength are observed for the paraffin filled with hydrogenated graphene. The results clearly show that the hydrogenation of graphene exerts opposite effects on the thermal and mechanical properties of graphene–paraffin nanocomposites. Thus hydrogenation is suggested to be used wisely in the graphene–paraffin nanocomposite so as to improve its interfacial thermal conductance at the minimum cost of its mechanical strength.

 Please wait while we load your content...
Please wait while we load your content...