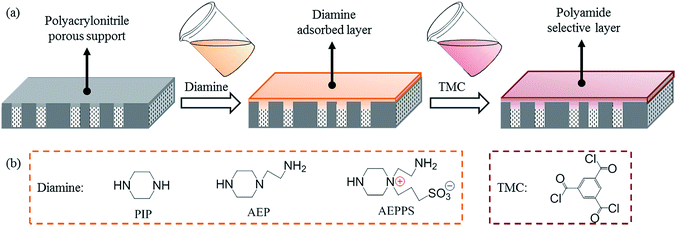
Tailoring the structure of polyamide thin film composite membrane with zwitterions to achieve high water permeability and antifouling property
Xiaodan Wengb,
Yanli Jia,
Fengyang Zhaoa,
Quanfu An*a and
Congjie Gaobc
aMOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science & Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: anqf@zju.edu.cn
bCollege of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, China
cThe Development Center of Water Treatment Technology, Hangzhou 310012, China
First published on 12th November 2015
Abstract
A series of carefully designed polyamide thin film composite nanofiltration membranes (TFCMs) were prepared via interfacial polymerization of piperazine (PIP), N-aminoethyl piperazine (AEP) or N-aminoethyl piperazine propane sulfonate (AEPPS) with trimesoyl chloride (TMC) on top of hydrolysed polyacrylonitrile ultrafiltration supporting membranes (hPAN-UF). Chemical structures of the TFCMs were evaluated by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) and X-ray photoelectron spectroscopy (XPS). The cross section and surface characteristics of the TFCMs were examined by scanning electron microscopy (SEM), atomic force microscopy (AFM), water contact angle measurements and zeta potential. Membrane fouling characteristics were studied by the adsorption of bovine serum albumin (BSA) and lysozyme (LYZ). Varying the diamine affected the structure of the resulting membranes, which ultimately defined separation performance and antifouling property. The results of water contact angle indicated zwitterionic membranes were more hydrophilic, which incorporated polyamide selective layer thickness to account for their high water flux (80.3 L m−2 h−1). Compared with other TFCMs, zwitterionic membranes showed an improved antifouling property due to their high hydrophilicity, low surface electrical charges and smooth surface roughness. These results provide important insights into the high water permeability and low fouling characteristics of zwitterionic nanofiltration membranes from molecular structure and interfacial polymerization process.
Introduction
One of the most pervasive problems afflicting people throughout the world is inadequate access to clean water and sanitation.1 In this regard membrane technologies such as nanofiltration and desalination are emerging as promising resolution to water problems owing to its low cost, energy efficiency and environmental-friendly characters.1–3 Since the breakthrough discovery made by Cadotte in the 1970s, polyamide thin film composite membranes (TFCMs) prepared using interfacial polymerization technique have experienced significant progress and emerged as the state-of-the-art technology in freshwater production and wastewater treatment processing.4,5 However, membrane fouling is a notorious practical problem ubiquitously encountered by separation membranes.6,7 It severely reduces the separation efficacy and increases the running cost.8 The fouling could be minimized if chlorine or other oxidants were added to the feed, but it poses environmental risks to aquatic life and human health when treated water is discharged back into the environment.9 Hence, there is a need for advanced membranes that have higher permeability and are less susceptible to various foulants, to deal with a growing array of practical applications.10Currently, great efforts have been taken to tailor the structures of TFCMs to improve their water permeability and/or antifouling properties.11 Examples included the modification of polyamide membranes achieved by the incorporation of functionalized organic comonomers12 or inorganic additives.13,14 However, the unsurpassed separation performance of polyamide limits the range of improvements that can be made following this pathway. Other studies focused on the membrane surface engineering by surface modification15–17 or improvement of the interfacial polymerization process.18,19 Unfortunately, these surface engineered membranes so far are not easy to operate in off-the-shelf manufacture processes. Apart from those routes mentioned above, selecting or synthesizing novel monomers with designed properties and preparing TFCMs directly tailored physicochemical properties of the membranes thoroughly.20 Zhang et al.21 synthesized tetra-functional biphenyl acid chloride and used as new monomers for the preparation of the TFCMs through interfacial polymerization with m-phenylenediamine (MPD). After that, Freeman et al.22 synthesized disulfonated bis[4-(3-aminophenoxy)-phenyl]sulfone and used in place of MPD to prepare TFCMs successfully, which had higher permeate flux than MPD/trimesoyl chloride (TMC) membranes. All of the above studies have showed that the membrane permeability was improved by using new monomers. Nevertheless, the salt retention of these polyamide membranes was compromised and the antifouling property was not assessed.
Zwitterions as a new type of molecules with high hydrophilicity and antifouling property are becoming a promising material for nanofiltration membranes. Ji et al.23 obtained novel composite nanofiltration membranes with enhanced flux and antifouling property by introducing zwitterionic terpolymers into nanofiltration membranes via the surface coating and chemical cross-linking methods. Chen et al.24 showed similar results by grafting poly(sulfobetaine methacrylate) from the surface of polyamide membranes. Recently, An et al. designed a new type of zwitterionic diamine monomer, N-aminoethyl piperazine propane sulfonate (AEPPS) and prepared the modified TFCMs via incorporating AEPPS as a functionalized diamine comonomer25 or 2-step interfacial polymerization.26 The water permeability and antifouling property of the modified TFCMs were improved by introducing AEPPS into membranes. However, because of the low diffusivity and reactivity of AEPPS, it is difficult to obtain a defect-free zwitterionic polyamide nanofiltration membrane prepared with the zwitterionic monomer directly.
In this work, a series of carefully designed polyamide thin film composite nanofiltration membranes with similar chemical structure were prepared on the top of hydrolysed polyacrylonitrile ultrafiltration supporting membranes (hPAN-UF) via interfacial polymerization with diamines and TMC. The diamines including piperazine (PIP), N-aminoethyl piperazine (AEP) and AEPPS were specifically chosen to have the similar structure but with different solubility parameters (δ). Effects of the diamine structure on the morphology and structure of the resulting polyamide membranes were investigated. In addition, effect of the physicochemical properties such as surface roughness, hydrophilicity, electrical charge and thickness on the separation performance and antifouling property were also studied.
Experimental
Materials
PIP (99%), AEP (99%), TMC (98%), bromophenol blue (BPB, molecular weight 670 Da, 98%) and congo red (CR, molecular weight 697 Da, 98%) were purchased from TCI (Shanghai) Development Co., Ltd and used as received. Sodium hydroxide (NaOH), sodium chloride (NaCl), sodium sulfate (Na2SO4), polyethylene glycol (PEG, molecular weight 600/1000 Da), toluene and acetonitrile were obtained from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. NaOH, NaCl, Na2SO4 and toluene were all analytical grade and used without any further purification. Acetonitrile was purified to anhydrous before used. 1,3-Propanesultone (1,3-PS, 98%), phosphate-buffered saline (PBS, pH 7.4, BioPerformance Certified), bovine serum albumin (BSA, molecular weight 67 kDa, isoelectric point 4.7, 98%), lysozyme (LYZ, molecular weight 14.3 kDa, isoelectric point 11.0, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mg−1) and glutaraldehyde solution (GA, specially purified for use as an electron microscopy fixative, 25%) were obtained from Sigma-Aldrich Co., Ltd. Deionized water (18 MΩ cm) was used in all experiments. Pristine polyacrylonitrile ultrafiltration supporting membranes (PAN-UF, molecular weight cut-off 30 kDa) were supplied by Shanghai Lanjing membrane technology Co., Ltd and hydrolysed in 5 wt% NaOH at 50 °C for 30 min before use. In addition, the water flux and retention performance of hPAN-UF were tested (pure water flux 303 L m−2 h−1 bar−1, molecular weight cut-off 30 kDa, retention to NaCl 0.4%, retention to Na2SO4 3.5%, retention to PEG600 15.0%, retention to PEG1000 22.0%).
000 U mg−1) and glutaraldehyde solution (GA, specially purified for use as an electron microscopy fixative, 25%) were obtained from Sigma-Aldrich Co., Ltd. Deionized water (18 MΩ cm) was used in all experiments. Pristine polyacrylonitrile ultrafiltration supporting membranes (PAN-UF, molecular weight cut-off 30 kDa) were supplied by Shanghai Lanjing membrane technology Co., Ltd and hydrolysed in 5 wt% NaOH at 50 °C for 30 min before use. In addition, the water flux and retention performance of hPAN-UF were tested (pure water flux 303 L m−2 h−1 bar−1, molecular weight cut-off 30 kDa, retention to NaCl 0.4%, retention to Na2SO4 3.5%, retention to PEG600 15.0%, retention to PEG1000 22.0%).
Synthesis of zwitterionic diamine monomer AEPPS
AEPPS was synthesized by the ring opening reaction of 1,3-PS with AEP according to the method reported in our previous publication.25 Typically, 7.22 g AEP dissolved in 60 mL acetonitrile was added into a 250 mL flask equipped with a stirrer and a thermometer. Then a mixture of 5.9 g 1,3-PS and 20 mL acetonitrile was added dropwise into the flask at 30 °C for 60 min. Afterwards the reaction was conducted at 30 °C for 6 h. After being cooled down, a light yellow crude product was collected by filtration. Then it was washed thoroughly with acetonitrile to remove unreacted reagents, and further purified by repeated dissolution in water and precipitation in acetonitrile. The product was dried in a vacuum oven to a constant weight at 50 °C. Consequently, the yield of the product AEPPS was about 75%.Preparation of the TFCMs
As depicted in Fig. 1, the TFCMs were prepared by interfacial polymerization of polyamide onto hPAN-UF. The hPAN-UF was immersed in a 0.080 mol L−1 PIP, AEP or AEPPS aqueous solution for 5 min, and the excess solution was removed from the membrane surface. Next, the diamine-saturated support membrane was immersed in a 0.010 mol L−1 TMC in toluene for 2 min, to form the ultrathin polyamide selective layer by interfacial polymerization. After removing the excess organic solution, the resulting membrane was cured at 60 °C for 15 min to carry out further polymerization. Finally, the prepared TFCM-1 (PIP-TMC TFCM), TFCM-2 (AEP-TMC TFCM) and TFCM-3 (AEPPS-TMC TFCM) were washed thoroughly with water and then stored in water before tests. Herein, the concentration of diamine and TMC was selected as 0.080 mol L−1 and 0.010 mol L−1 to keep the cross-linking extent of TFCMs analogous.Membrane characterization and performance assessment
All the membrane samples were flushed with water and dried thoroughly at 25 °C under vacuum for 24 h prior to characterization. Chemical structures of TFCMs were determined by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR, Nicolet 6700, Thermo Fisher Scientific, USA) with a ZnSe crystal as the internal reflection element with an angle of incidence of 45°. Elemental content of TFCMs was evaluated from X-ray photoelectron spectroscopy (XPS, Escalab 250Xi, Thermo Fisher Scientific, USA). The cross section and surface morphologies of the TFCMs were visualized by field emission scanning electron microscopy (FESEM, S4800, HITACHI, Japan). TFCMs were fractured in liquid nitrogen to examine their cross section structures. SEM samples were sputter-coated with gold and analyzed at a voltage of 3 kV. Membrane surface roughness of TFCMs was analyzed using a multimode atomic force microscopy (AFM, SPI3800N, Seiko Instruments Inc., Japan) in tapping mode at room temperature in air atmosphere. The surface roughness of each membrane was quantified as the root mean-square (RMS) roughness measured from height profile of three-dimensional AFM images.Surface hydrophilicity of TFCMs was characterized by dynamic water contact angle measurements (OCA 20, Data Physics Instruments GmbH, Germany) using the sessile drop method at ambient temperature. The dynamic water contact angle was determined from a magnified image of the water droplet taken by a digital camera and analyzed using the DROPimage software. The equilibrium value was the steady-state average of left and right angles. The highest and the lowest equilibrium angles tested in five random locations were discarded and the remaining data were averaged.
The effective surface charge of TFCMs was evaluated from zeta potential measurements (EKA, Anton Paar GmbH, Austria). Zeta potential measurements were carried out with 0.001 mol L−1 KCl aqueous solutions at 25 ± 1.0 °C and pH ranging from 3.5 to 9.5. Surface zeta potential was determined from the Helmholtz–Smoluchowski equation with the Fairbrother and Mastin substitution.27 The data presented are average values from three samples of each membrane type.
Nanofiltration performance in terms of water flux and solute retention of TFCMs was investigated with a cross-flow flat apparatus with three parallel circular filtration cells at 25 °C, 0.6 MPa and pH 7.0 if not specified. The membranes were pre-filtrated with water at 0.6 MPa to reach a steady state before testing. Then the water flux and solute retention were measured with 1 g L−1 inorganic salts and 0.1 g L−1 dyes aqueous solution. Between each different feed solution, the membranes were washed thoroughly with deionized water. After completing the tests, all membranes were rinsed cleanly with deionized water.
The water flux (J) and solute retention (R) were calculated by the following eqn (1) and (2):
 | (1) |
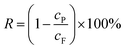 | (2) |
Protein adsorption behaviors of TFCMs were examined by a similar method to that reported in the literature.28 BSA/LYZ solutions were prepared by slowly dissolving preweighed amounts of protein powder in 0.01 mol L−1 PBS solution. All protein solutions were filtered through 0.22 μm syringe filters immediately prior to use. Protein solutions were stored at 4 °C and used within 24 h of preparation to minimize protein aggregation or denaturation. A single membrane sample with an area of 5.0 cm2 was placed in 10 mL 1 g L−1 protein solution and allowed to soak for 24 h. After protein adsorption experiment, the membranes were cleaned with 0.01 mol L−1 PBS solution for three times, dried thoroughly at 25 °C under vacuum, coated with gold powder by a vacuum electric sputter coater and observed with FESEM (S4800, HITACHI, Japan) for their surface images. In addition, the protein concentration in the solution was determined from UV-vis spectrophotometer (TU-1810PC). The change in protein concentration was used to calculate the amount of protein adsorbed on the membrane by a simple mass balance.
Calculation of solubility parameter
The separation performance of polyamide membranes is usually influenced by the solubility parameter of the two immiscible solutions in interfacial polymerization.29,30 The solubility parameters of diamine monomers for TFCMs were calculated as following:For an uncharged diamine, such as PIP and AEP, the solubility parameter was calculated by the following eqn (3):31,32
 | (3) |
For a charged diamine, such as AEPPS, the solubility parameter was calculated by the following eqn (4):33
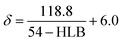 | (4) |
Results and discussion
Chemical structures and compositions of TFCMs
The chemical structures and compositions of the TFCMs were characterized by ATR-FTIR and XPS. Fig. 2 presents ATR-FTIR spectra of hPAN-UF, TFCM-1, TFCM-2, TFCM-3 and FTIR spectrum of AEPPS, respectively. For the spectrum of hPAN-UF, the characteristic peak appearing at 2242 cm−1 is assigned as the stretching vibration of cyano groups (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) on hPAN-UF. The largest peak for TFCM-1, attributed to the amide I (C
N) on hPAN-UF. The largest peak for TFCM-1, attributed to the amide I (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretch, appears at 1621 cm−1. The characteristic peaks appearing at 1025 cm−1 and 1010 cm−1 are attributed to the tertiary amine groups (
O) stretch, appears at 1621 cm−1. The characteristic peaks appearing at 1025 cm−1 and 1010 cm−1 are attributed to the tertiary amine groups (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–) on polyamide matrix. Compared with TFCM-1, a new peak at 1553 cm−1 appears in the spectrum of TFCM-2, which is ascribed to the amide II (N–H) bending vibration. Apart from the above mentioned characteristic peaks, the peak at 1042 cm−1 for sulfonic acid group of AEPPS was observed for TFCM-3.25 This confirms that a new kind of zwitterionic polyamide membrane was successfully prepared with AEPPS and TMC on the hPAN-UF supporting membrane via interfacial polymerization. Furthermore, the elemental composition of TFCMs was investigated with XPS analysis. As showed in Table 1, zwitterion content on TFCM-3 surface is 58.4%, which is significantly higher than those reported in the literatures.23–26 The cross-linking extent of TFCM-1, TFCM-2 and TFCM-3 was comparable and was 83.8%, 82.8% and 80.7%, respectively.
N–) on polyamide matrix. Compared with TFCM-1, a new peak at 1553 cm−1 appears in the spectrum of TFCM-2, which is ascribed to the amide II (N–H) bending vibration. Apart from the above mentioned characteristic peaks, the peak at 1042 cm−1 for sulfonic acid group of AEPPS was observed for TFCM-3.25 This confirms that a new kind of zwitterionic polyamide membrane was successfully prepared with AEPPS and TMC on the hPAN-UF supporting membrane via interfacial polymerization. Furthermore, the elemental composition of TFCMs was investigated with XPS analysis. As showed in Table 1, zwitterion content on TFCM-3 surface is 58.4%, which is significantly higher than those reported in the literatures.23–26 The cross-linking extent of TFCM-1, TFCM-2 and TFCM-3 was comparable and was 83.8%, 82.8% and 80.7%, respectively.
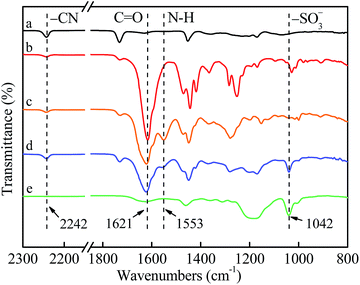 | ||
| Fig. 2 ATR-FTIR spectra of (a) hPAN-UF, (b) TFCM-1, (c) TFCM-2, (d) TFCM-3 and (e) FTIR spectrum of AEPPS. | ||
| Membranes | Atomic conc. (mol%) | Cross-linking extenta | Diamine contenta | |||
|---|---|---|---|---|---|---|
| C (1s) | N (1s) | O (1s) | S (2p) | |||
a Cross-linking extent (x) and diamine content of TFCMs was calculated by the eqn (5) and (6) on the basis of C (1s) and N (1s) atomic concentration determined by XPS.   where m and n are the number of C and N in diamine chemical formulas, respectively. where m and n are the number of C and N in diamine chemical formulas, respectively. |
||||||
| TFCM-1 | 69.35 | 13.41 | 17.24 | — | 83.8% | 58.7% |
| TFCM-2 | 67.72 | 16.43 | 15.85 | — | 82.8% | 58.6% |
| TFCM-3 | 64.84 | 12.62 | 19.93 | 2.61 | 80.7% | 58.4% |
Surface and cross-section characteristics of TFCMs
The membrane morphologies would be tuned well with the variation of monomer chemical structures. As showed in Fig. 3, the polyamide selective layer thickness of TFCM-3 is about 110 nm, which is much thinner than that of TFCM-1 (210 nm) and TFCM-2 (320 nm). Furthermore, both TFCM-1 and TFCM-3 show a uniform grainy morphology, which is similar as most of polyamide thin films formed by interfacial condensation.4 While, there are the much different images for TFCM-2, which exhibit a uniform smooth morphology and low RMS value. Compared with the porous hPAN-UF surface, the membrane surface of TFCM-3 exhibits no appreciable pores or cracks, indicating a defect-free dense polyamide selective layer was formed on the hPAN-UF. | ||
| Fig. 3 Cross-section (×50.0k) of (a) hPAN-UF, (b) TFCM-1, (c) TFCM-2 and (d) TFCM-3; SEM (×50.0k)/AFM surface morphologies of (e)/(i) hPAN-UF, (f)/(j) TFCM-1, (g)/(k) TFCM-2 and (h)/(l) TFCM-3. | ||
These changes in membrane thickness and surface morphology are attributed to the difference between PIP, AEP and AEPPS in solubility parameters.21,30 For film thickness to build up, diamine contained in water must continually cross the water–toluene interface, diffuse through the polyamide layer already formed, then come into contact with TMC on the organic solvent side of the polyamide selective layer.4 Both partition of diamine and its solubility in toluene influence the morphology and thickness of the TFCMs.36 Because both PIP and AEPPS are insoluble in toluene, there are concentration fluctuations for them in toluene, resulting in a grainy morphology and high surface roughness.36–38 AEPPS is difficult to partition into toluene phase because the solubility parameter of AEPPS (15.85 cal1/2 cm−3/2) is very different from the solubility parameter of toluene (δ = 8.9 cal1/2 cm−3/2). That is why the polyamide selective layer of the resultant TFCM-3 is thin. Furthermore, the low diffusivity of AEPPS limits concentration fluctuations and as a result leads to a smoother morphology. However, PIP is prone to partition into toluene phase with a solubility parameter of 11.0 cal1/2 cm−3/2, which is much closer to the solubility parameter of toluene. The polyamide layer of the resultant TFCM-1 is thicker. As the content of PIP in toluene increases, the concentration fluctuations become wilder. It results to an obvious grainy morphology and an increased RMS roughness. However, AEP is different from PIP and AEPPS, which is soluble in toluene with a solubility parameter of 9.4 cal1/2 cm−3/2. There are no concentration fluctuations for AEP in toluene, which results in a smooth morphology. However, the high concentration of AEP in toluene leads to a high thickness selective layer.
Both of the hydrophilicity and surface charge of membranes are critical for separation performances in nanofiltration process. The dynamic water contact angle was determined to investigate the hydrophilicity of hPAN-UF and TFCMs (Fig. 4(a)). The initial contact angle of hPAN-UF is 33.0° and decreases to less than 5° quickly, which is probably due to the hydrophilic and porous surface of hPAN-UF. The water contact angle of TFCM-3 is 32.7°, which is much smaller than that of TFCM-1 (49.6°) and TFCM-2 (66.4°). The high hydrophilicity of TFCM-3 is due to the large number of zwitterionic groups on AEPPS,39,40 whose solubility parameter is closest to that of water. And it is beneficial for water molecules to form a tightly bound water layer.41,42 This is a desirable trend because a superhydrophilic surface is a significant property in terms of good membrane permeability and antifouling.40,43 Moreover, the effective surface charge of TFCMs was evaluated with zeta potential measurements as showed in Fig. 4(b). The zeta potential of TFCMs decreases with increasing the pH value. The TFCMs are positively charged in acid aqueous solutions with a low pH value due to the adsorption of hydrogen ions. However, more carboxylic acid groups of TFCMs were deprotonated, and more hydroxide ions were adsorbed onto the membrane surface in alkaline aqueous solutions, resulting in a much higher negatively charged density of membranes.25 In addition, the isoelectric point of TFCM-3 was pH 4.7 and higher than that of TFCM-1 (pH 4.1). This is probably attributed to the shadowing effect of electrically neutral zwitterionic AEPPS.44 Moreover, the isoelectric point of TFCM-2 (pH 5.4) is much higher than that of TFCM-3. This is because the tertiary amine group in TFCM-2 was quaternarized in acid aqueous solutions, which leads to a higher positively charged density for the membrane. Thus, the zeta potential of TFCM-2 increases significantly with decreasing the pH value.
 | ||
| Fig. 4 (a) Dynamic water contact angle of hPAN-UF, TFCM-1, TFCM-2 and TFCM-3 and (b) zeta potential varies with pH of TFCM-1, TFCM-2 and TFCM-3 tested with 1.0 mM KCl aqueous solution at 25 °C. | ||
Separation performance of TFCMs
The separation performance of as-prepared membranes was investigated with 1 g L−1 NaCl, Na2SO4, PEG600 or PEG1000 aqueous solutions. As showed in Fig. 5(a), the water flux through TFCM-3 at 25 °C and 0.6 MPa is 80.3 L m−2 h−1, which is 3.8 and 8.1 times of the flux of TFCM-1 (21.0 L m−2 h−1) and TFCM-2 (9.9 L m−2 h−1), respectively. The increase in water permeability is probably attributed to the thin polyamide selective layer and hydrophilic surface resulting from high AEPPS content on membrane surface. As showed in Fig. 5(b), when tested with 1 g L−1 PEG600 and PEG1000 aqueous solutions, the average retention of TFCM-3 to PEG600 and PEG1000 is about 92.8% and 98.5%, respectively. Aside from the successful retention to PEG600 and PEG1000, the average retention of TFCM-3 to Na2SO4 and NaCl is relatively low, 78.1% and 14.3%, respectively. Consequently, TFCM-3 with higher water permeability and organic/salts selectivity are suitable for concentrating or separating the organic substances in industry and environment.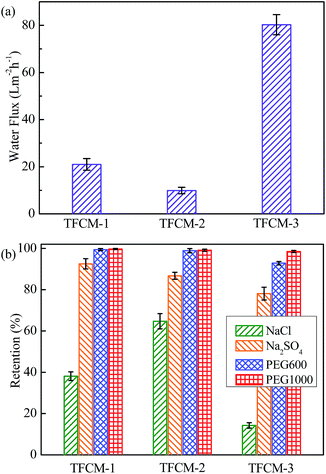 | ||
| Fig. 5 Nanofiltration performance of TFCM-1, TFCM-2 and TFCM-3 tested with 1 g L−1 NaCl, Na2SO4, PEG600 or PEG1000 aqueous solutions at 25 °C under 0.6 MPa. | ||
Synthetic dyes are widely used in textile manufacturing processes. However, they are difficult to be biodegraded in environment because of their complex aromatic molecular structures.45,46 Fortunately, nanofiltration technique is an effective membrane process to separation and removal of dyes in industry and environment. In this article, we take bromophenol blue and congo red as model dyes to study the application of TFCM-3 for dyes separation. Fig. 6 shows the water flux, salt and dye retention of the TFCM-3 when TMC concentration in toluene phase increases from 0.0025 to 0.03 mol L−1. With increasing the TMC concentration from 0.0025 to 0.01 mol L−1, water flux decreases to 80.3 L m−2 h−1, and the average retention to BPB and CR increases to the maximum, 93.8% and 97.0%, respectively. With increasing the TMC concentration, more TMC involves in interfacial polymerization, which generates a more compact polyamide layer.30 As the cross-linking extent increases, the water flux decreases and dye retention increases. However, with the further increasing TMC concentration from 0.01 to 0.03 mol L−1, the cross-linking extent decreases, leading to an increase in flux and a decrease in retention.30
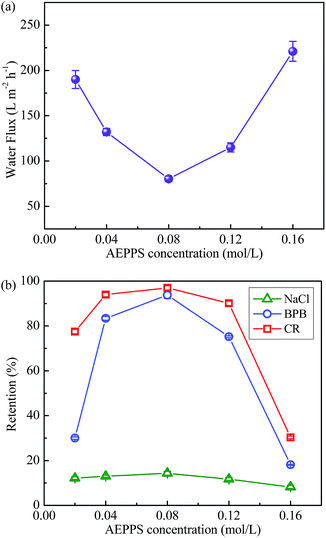
Subsequently, the TMC concentration was fixed at 0.01 mol L−1, the influence of AEPPS concentration on the separation performance of TFCM-3 was investigated. As showed in Fig. 7, the water flux first decreases and then increases with increasing the AEPPS concentration, and vice versa for the rejection. The turning point is at 0.08 mol L−1 AEPPS concentration with a flux of 80.3 L m−2 h−1. The average retention to BPB and CR reaches the maximum, 93.8% and 97.0%, respectively. With increasing the AEPPS concentration from 0.02 to 0.08 mol L−1, more AEPPS would diffuse through the nascent polyamide layer and react with TMC in organic phase, resulting in a much dense selective layer.4,36 However, with the further increasing AEPPS concentration, the cross-linking extent could decreases, which is probably induced by the blocking reaction of the excessive AEPPS.30 Consequently, the cross-linking extent of the membranes decreases with decreasing/increasing the AEPPS concentration, leading to an increase in flux and a decrease in retention. Notably, the retention to CR is somewhat higher than that to BPB. This is attributed to the electrostatic repulsion between the negatively charged congo red and the membrane surface. Aside from the variable retention to dyes, the retention of TFCM-3 to NaCl maintains lower than 15%, which is suitable for dyes/salt separation. These results demonstrate that the separation performance of TFCM-3 could be tuned with adjusting the TMC and AEPPS concentration in interfacial polymerization.
Antifouling property of TFCMs
Apart from the exceptional separation capability, TFCM-3 is also expected to have good antifouling performance, which is very important in applications because the membrane fouling severely reduces the separation efficacy and increases the running cost.9,47,48 Fig. 8 shows the SEM surface images of hPAN-UF and TFCMs and the amount of protein adsorbed onto the membrane surface after immersion in 1.0 g L−1 BSA or LYZ aqueous solution at pH 7.4 for 24 h. At such a pH value, BSA is negatively charged (∼−22.3 mV) and LYZ is positively charged (∼+3.5 mV).49 As showed in Fig. 8, there is some BSA foulant adsorbed on the hPAN-UF surface (40 μg cm−2). However, it is obviously seen that there is much more BSA foulant adsorbed on the TFCM-1 surface (134 μg cm−2) than that on TFCM-2 (96 μg cm−2). Most of studies have demonstrated that the smoother membrane surface is less susceptible to be attacked with foulant.24,47 Thus TFCM-2 with much smooth membrane surface exhibits a better resistance to protein adsorption. Furthermore, there is very little BSA adsorbed on the zwitterionic TFCM-3 surface (11 μg cm−2). This is because TFCM-3 has a moderate smooth surface, which is benefit to resist the protein fouling. Moreover, the zwitterionic TFCM-3 is highly hydrophilic, the hydration layer formed on the membrane prevents protein foulant contact with the membrane surface, resulting in a significant antifouling performance.40,44,50–53 In addition, as showed in Fig. 8(a′–d′), much more LYZ foulant was adsorbed onto the membrane surface of hPAN-UF, TFCM-1 and TFCM-2, resulting from the strong electrostatic attractions between the positively-charged LYZ and the negatively-charged membrane surface. However, TFCM-3 still exhibits an outstanding antifouling performance. Furthermore, the flux decline ratio of hPAN-UF and TFCMs after BSA and LYZ adsorption on the membranes was investigated for comparing the antifouling property of these membranes in water treatment application. As seen in Fig. 9, the flux decline ratio of TFCM-3 after BSA adsorption on the membrane is about 2.0%, which is much lower than that of hPAN-UF (24.5%), TFCM-1 (28.2%) and TFCM-2 (18.7%), respectively. Moreover, choosing LYZ as the model foulant, the flux decline ratio of hPAN-UF and TFCMs is in the same variation trend, while which is a little higher than that testing with BSA due to the stronger electrostatic interaction. All of these above results clearly demonstrate that the strong antifouling property of zwitterionic TFCM-3 is strongly dependent on the surface characteristics of the membrane, including the low surface roughness, high hydrophilicity and charged density.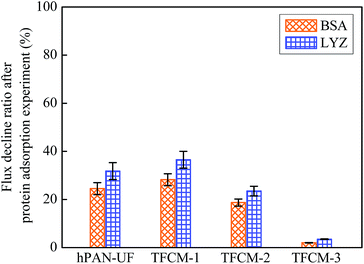 | ||
| Fig. 9 Flux decline ratio of hPAN-UF, TFCM-1, TFCM-2 and TFCM-3 after BSA and LYZ adsorption on the membranes. | ||
Conclusions
TFCM-3 has been prepared through interfacial polymerization of AEPPS with TMC on the top of hPAN-UF. Effects of the zwitterion on the structure, separation performance and antifouling property of the resulting polyamide membranes are investigated by comparing a series of TFCMs with similar chemical structure. The difference between PIP, AEP and AEPPS in solubility parameters attributes to the changes in membrane thickness and surface morphology, which influences water permeability and antifouling property of the membranes. The water flux of TFCM-3 is 80.3 L m−2 h−1, which is 3.8 and 8.1 times as that of TFCM-1 and TFCM-2, respectively. TFCM-3 is highly hydrophilic, which incorporates polyamide selective layer thickness to account for its remarkably high flux. The zwitterionic membranes also exhibit a better protein adsorption resistance, including conditions where the protein is positively-charged (LYZ) or negatively-charged (BSA). The antifouling property of the membranes is strongly dependent on the characteristics of the membrane surfaces, including the surface charges, hydrophilicity and roughness. It seemed that all the difference containing: the membrane structure, hydrophilicity, separation and antifouling performance, might come from the different structure and reactivity of three diamine monomers. These results provide important insights into the high water flux and low fouling characteristics of zwitterionic nanofiltration membranes from molecular structure and interfacial polymerization process.Acknowledgements
This research was financially supported by Zhejiang Province Natural Science Foundation (No. LR15B060001), National Basic Research Program of China (No. 2015CB655303), National Natural Science Foundation of China (No. 21306163) and Zhejiang University K. P. Chao's High Technology Development Foundation.Notes and references
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nature, 2008, 452, 301 CrossRef CAS PubMed
.
- M. G. Buonomenna, RSC Adv., 2013, 3, 5694 RSC
.
- B. van der Bruggen and C. Vandecasteele, Environ. Pollut., 2003, 122, 435 CrossRef CAS PubMed
.
- R. J. Petersen, J. Membr. Sci., 1993, 83, 81 CrossRef CAS
.
- N. Misdan, W. J. Lau and A. F. Ismail, Desalination, 2012, 287, 228 CrossRef CAS
.
- D. Rana and T. Matsuura, Chem. Rev., 2010, 110, 2448 CrossRef CAS PubMed
.
- Y. P. Tang, J. X. Chan, T. S. Chung, M. Weber, C. Staudt and C. Maletzko, J. Mater. Chem. A, 2015, 3, 10573 CAS
.
- Y. F. Li, Y. L. Su, X. T. Zhao, X. He, R. N. Zhang, J. J. Zhao, X. C. Fan and Z. Y. Jiang, ACS Appl. Mater. Interfaces, 2014, 6, 5548 CAS
.
- M. Elimelech and W. A. Phillip, Science, 2011, 333, 712 CrossRef CAS PubMed
.
- G. M. Geise, H. S. Lee, D. J. Miller, B. D. Freeman, J. E. Mcgrath and D. R. Paul, J. Polym. Sci., Part B: Polym. Phys., 2010, 48, 1685 CrossRef CAS
.
- G. D. Kang and Y. M. Cao, Water Res., 2012, 46, 584 CrossRef CAS PubMed
.
- A. L. Ahmad, B. S. Ooi and J. P. Choudhury, Desalination, 2003, 158, 101 CrossRef CAS
.
- H. Huang, X. Qu, H. Dong, L. Zhang and H. Chen, RSC Adv., 2013, 3, 8203 RSC
.
- Q. Li, Y. Wang, J. Song, Y. Guan, H. Yu, X. Pan, F. Wu and M. Zhang, Appl. Surf. Sci., 2015, 324, 757 CrossRef CAS
.
- E. M. van Wagner, A. C. Sagle, M. M. Sharma, Y. H. La and B. D. Freeman, J. Membr. Sci., 2011, 367, 273 CrossRef CAS
.
- A. Tiraferri, Y. Kang, E. P. Giannelis and M. Elimelech, ACS Appl. Mater. Interfaces, 2012, 4, 5044 CAS
.
- D. Saeki, S. Nagao, I. Sawada, Y. Ohmukai, T. Maruyama and H. Matsuyama, J. Membr. Sci., 2013, 428, 403 CrossRef CAS
.
- H. Zou, Y. Jin, J. Yang, H. J. Dai, X. L. Yu and J. Xu, Sep. Purif. Technol., 2010, 72, 256 CrossRef CAS
.
- T. Tsuru, S. Sasaki, T. Kamada, T. Shintani, T. Ohara, H. Nagasawa, K. Nishida, M. Kanezashi and T. Yoshioka, J. Membr. Sci., 2013, 446, 504 CrossRef CAS
.
- Y. C. Chiang, Y. Z. Hsub, R. C. Ruaan, C. J. Chuang and K. L. Tung, J. Membr. Sci., 2009, 326, 19 CrossRef CAS
.
- L. Li, S. B. Zhang, X. S. Zhang and G. D. Zheng, J. Membr. Sci., 2008, 315, 20 CrossRef CAS
.
- W. Xie, G. M. Geise, B. D. Freeman, H. Lee, G. Byun and J. E. McGrath, J. Membr. Sci., 2012, 403–404, 152 CrossRef CAS
.
- Y. L. Ji, Q. F. An, Q. Zhao, W. D. Sun, K. R. Lee, H. L. Chen and C. J. Gao, J. Membr. Sci., 2012, 390, 243 CrossRef
.
- Y. Zhang, Z. Wang, W. F. Lin, H. T. Sun, L. G. Wu and S. F. Chen, J. Membr. Sci., 2013, 446, 164 CrossRef CAS
.
- Q. F. An, W. D. Sun, Q. Zhao, Y. L. Ji and C. J. Gao, J. Membr. Sci., 2013, 431, 171 CrossRef CAS
.
- Y. F. Mi, Q. Zhao, Y. L. Ji, Q. F. An and C. J. Gao, J. Membr. Sci., 2015, 490, 311 CrossRef CAS
.
- A. E. Childress and M. Elimelech, J. Membr. Sci., 1996, 119, 253 CrossRef CAS
.
- M. Hadidi and A. L. Zydney, J. Membr. Sci., 2014, 452, 97 CrossRef CAS
.
- M. Hirose and K. Ikeda, US Pat., 5576057, 1996
.
- A. K. Ghosh, B. Jeong, X. Huang and E. M. V. Hoek, J. Membr. Sci., 2008, 311, 34 CrossRef CAS
.
- K. L. Hoy, J. Paint Technol., 1970, 42, 76 CAS
.
- R. F. Fedors, Polym. Eng. Sci., 1974, 14, 147 CAS
.
- R. C. Little, J. Colloid Interface Sci., 1978, 65, 587 CrossRef CAS
.
- J. T. Davies, A quantitative kinetic theory of emulsion type I: Physical chemistry of the emulsifying agent, Butterworths Scientific Publications, London, 1957 Search PubMed
.
- J. T. Davies and E. K. Rideal, Interfacial phenomena, Academic Press, New York, 1961 Search PubMed
.
- H. F. Wang, Q. F. Zhang and S. B. Zhang, J. Membr. Sci., 2011, 378, 243 CrossRef CAS
.
- V. Freger, Langmuir, 2005, 21, 1884 CrossRef CAS PubMed
.
- S. Karan, Z. Jiang and A. G. Livingston, Science, 2015, 348, 1347 CrossRef CAS PubMed
.
- Y. C. Chiang, Y. Chang, C. J. Chuang and R. C. Ruaan, J. Membr. Sci., 2012, 389, 76 CrossRef CAS
.
- Q. F. An, Y. L. Ji, W. S. Hung, K. R. Lee and C. J. Gao, Macromolecules, 2013, 46, 2228 CrossRef CAS
.
- Z. Zhang, S. F. Chen, Y. Chang and S. Y. Jiang, J. Phys. Chem. B, 2006, 110, 10799 CrossRef CAS PubMed
.
- S. F. Chen, L. Y. Li, C. Zhao and J. Zheng, Polymer, 2010, 51, 5283 CrossRef CAS
.
- M. Ghanbari, D. Emadzadeh, W. J. Lau, T. Matsuura and A. F. Ismail, RSC Adv., 2015, 5, 21268 RSC
.
- A. Venault, H. S. Yang, Y. C. Chiang, B. S. Lee, R. C. Ruaan and Y. Chang, ACS Appl. Mater. Interfaces, 2014, 6, 3201 CAS
.
- V. R. S. S. Mokkapati, D. Y. Koseoglu Imer, N. Yilmaz, V. Ozguz and I. Koyuncu, RSC Adv., 2015, 5, 71011 RSC
.
- L. L. Shao, Q. F. An, Y. L. Ji, Q. Zhao, X. S. Wang, B. K. Zhu and C. J. Gao, Desalination, 2014, 338, 74 CrossRef CAS
.
- Q. Zhao, Y. L. Ji, J. K. Wu, L. L. Shao, Q. F. An and C. J. Gao, RSC Adv., 2014, 4, 52808 RSC
.
- H. Meng, Q. Cheng and C. Li, Appl. Surf. Sci., 2014, 303, 399 CrossRef CAS
.
- Y. H. Zhao, X. Y. Zhu, K. H. Wee and R. B. Bai, J. Phys. Chem. B, 2010, 114, 2422 CrossRef CAS PubMed
.
- R. Yang, H. Jang, R. Stocker and K. K. Gleason, Adv. Mater., 2014, 26, 1711 CrossRef CAS PubMed
.
- W. J. Yang, K. G. Neoh, E. T. Kang, S. L. M. Teo and D. Rittschof, Prog. Polym. Sci., 2014, 39, 1017 CrossRef CAS
.
- R. Bernstein, S. Belfer and V. Freger, Environ. Sci. Technol., 2011, 45, 5973 CrossRef CAS PubMed
.
- S. Rajabzadeh, R. Sano, T. Ishigami, Y. Kakihana, Y. Ohmukai and H. Matsuyama, Appl. Surf. Sci., 2015, 324, 718 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2015 |