Simple synthesis 11-substituted norcryptotackieine derivatives†
Michał Nowacki and
Krzysztof Wojciechowski*
Institute of Organic Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland. E-mail: kwojciechowski@icho.edu.pl
First published on 28th October 2015
Abstract
11-Substituted (CN, CO2Et, SO2Ph) indolo[2,3-b]quinolines were obtained in reactions of N-protected (SiMe2t-Bu, CO2t-Bu) indol-3-yl-acetonitrile, -acetate, and -methylsulfone with nitrobenzene derivatives in presence of base and trialkylchlorosilanes.
Introduction
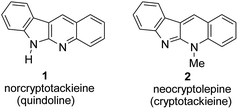
Norcryptotackieine (quinindoline, 6H-indolo[2,3-b]quinoline, 1) and neocryptolepine (cryptotackieine 2) are natural alkaloids isolated from Cryptolepis sanguinolenta, the plant used in traditional West African medicine for treatment of malaria.1,2 The planar structure of these alkaloids is a good starting platform for DNA-binding agents of potential anticancer activity.3The chemistry of 5H- and 6H-indolo[2,3-b]quinolines has been recently reviewed.4 Several methods of construction of this heterocyclic system have been developed, some of them were known5 before isolation of norcryptotackieine from natural sources. Selected starting materials that in a few steps can be transformed into indolo[2,3-b]quinoline framework are presented in the Scheme 1. These methods include reactions of 2-nitrobenzyltriphenylphosphonium bromide with isatin (a), indole with 2-nitrobenzylbromide (b),6 indole-3-carbaldehyde with aniline (c),7 2-chloroquinoline with 2-chloroaniline (d)8 or benzotriazole (e),9 2-iodo-3-bromoquinoline with aniline (f),10 2-nitrophenylacetic acid with 2-nitrobenzaldehyde (g),11 and 2-nitrobenzyl cyanide with 2-acetoxymethyl-2-cyclohexenone (h).12
In the course of our studies on reactions of nucleophilic substitution of hydrogen in nitroarenes we developed numerous transformations enabling direct synthesis of heterocycles, particularly indoles and quinolines, in reactions of carbanions with nitroarenes.13–16 The methods applicable to synthesis of quinolines consist of transformation of σH-adducts formed from nitroarenes and allyl carbanions generated from allyl sulfones17 or aminocrotonates.18 It was found that carbanions of arylylacetonitriles react with nitroarenes in the presence of Lewis acids or silylating agents to form 9-cyanoacridine derivatives (Scheme 2).19 Two variants of this methodology were developed. In the first one, all reagents: phenylacetonitrile, nitroarene, a relatively weak base such as DBU and a silylating agent (e.g. trimethylchlorosilane) or a Lewis acid (e.g. MgCl2) are mixed at once and stirred to complete the reaction. In the second protocol the mixture of carbanion precursor, nitroarene and a base (e.g. triethylamine) is treated at low temperature with a strong base such as t-BuOK. The formed carbanion adds to nitroarene to form σH-adduct that is then treated with a silylating agent and in the presence of the initially added base (triethylamine) creates pyridine ring yielding finally acridine.
It is worth mentioning that arylacetonitriles and nitroarenes in the presence of base in protic media transform into 3-aryl-2,1-benzisoxazoles (anthranils).20 We studied the effect of the reaction conditions on the competition between formation of acridines and anthranils from arylacetonitriles and nitroarenes.21 The reactions of nitroarenes with benzyl sulfone carbanions mediated by Lewis acid (MgCl2)22 or silylating agent21 led to 3-aryl-2,1-benzisoxazoles, instead of 9-(arenesulfonyl)acridines.
Results and discussion
In this paper we present application of reactions of indol-3-ylmethyl carbanions with nitrobenzene derivatives to synthesis of 11-substituted 6H-indolo[2,3-b]quinolines. As starting materials we have chosen indole derivatives 3 bearing at position 3 cyanomethyl, ethoxycarbonylmethyl, and benzenesulfonylmethyl groups. Since indole is a relatively strong N–H acid these derivatives were protected with t-butoxycarbonyl group.In our approach, potassium tert-butoxide was added to tetrahydrofuran solution containing N-(tert-butoxycarbonyl)indol-3-ylacetonitrile (3a, Z = CN), 4-chloronitrobenzene (4a) and triethylamine cooled to −70 °C. The generated in-situ carbanion reacts with the nitroarene to form σ-adduct, which was then treated with trimethylchlorosilane. The reaction mixture was then allowed to reach room temperature and stirred for 24 h giving after work-up indoloquinoline 5aa in 57% yield. Similarly reacted other nitrobenzene derivatives: 4-fluoronitrobenzene (4b), 4-bromonitrobenzene (4c) and 2,4-dichloronitrobenzene (4d). No formation of indoloquinoline was observed in reaction of nitrobenzene with indol-3-ylacetonitrile (3a, Z = CN). The carbanion precursor was consumed but complex mixture of products was formed arising probably from the σH-adduct formed at the para-position to the nitro group. Attempts to use of N-BOC protected ethyl indol-3-ylacetate (3b, Z = CO2Et) as carbanion precursor were partly successful. Its reaction with 4-bromonitrobenzene (4c) led to complex mixture of products from which after treatment of the reaction mixture with trifluoroacetic acid N-BOC deprotected indoloquinoline 5bc was isolated in 25% yield. In the reaction of the ester 3b with 2,4-dichloronitrobenzene (4d) the expected indoloquinoline 5bd was isolated in 11% yield (Scheme 3).
The plausible way of formation of indoloquinolines from σH-adduct A generated from N-protected indol-3-ylacetonitrile and nitroarenes is shown in Scheme 4. In the first step the σH-adduct A undergoes silylation and the formed silyl nitronate B is deprotonated to form intermediate C, that can be considered a product of replacement of hydrogen at the ortho-position to the nitro group in the original nitroarene by the carbanion moiety. Further deprotonation of C followed by silylation leads to bis-silylated intermediate D, which in turn eliminates silanol to form azaxylylene E. The electrocyclization of azaxylylene E leads to 5,5a-dihydroindoloquinoline framework F from which after elimination of another molecule of silanol the final product is formed. Similar electrocyclizations of aza-ortho-xylylenes leading to condensed heterocycles are known processes.23,24 The crucial point of the proposed mechanism is formation of bis-silylated intermediate D. There are some literature precedences of this type bis-silylated dihydroxylamines formed via double deprotonation/silylation of nitroalkenes. The most fitting example is N,N-bis-(tert-butyldimethylsilyloxy)aniline obtained in reaction of 1-nitro-1,3-cyclohexadiene with tert-butyldimethylsilyl triflate.25
 | ||
| Scheme 4 The proposed way of formation in reactions of indol-3-ylacetonitrile with nitroarenes mediated by trilakylchlorosilanes. | ||
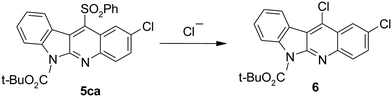
The reaction of N-BOC protected indol-3-yl sulfone (3c, Z = SO2Ph) with 4-chloronitrobenzene led after 24 h to 11-(benzenesulfonyl)indolo[2,3-b]quinoline (5ca) in 40% yield. However, when the reaction time was extended another product has appeared. After six days transformation of the initially formed benzenesulfonyl derivative 5ca was completed and 11-chloro derivative 6 was isolated. Formation of this product is a result of substitution of benzenesulfonyl group with chloride anions present in the reaction mixture (Scheme 5). Such replacement of arenesulfonyl group with various nucleophiles was earlier observed in 4-(arenesulfonyl)quinolines.26,27
The N-BOC protecting group can be easily removed from the formed products by treatment with trifluoroacetic acid. This approach was used for the product formed from the sulfone 3c and 4-bromonitrobenzene (4c) giving the product 5cc.
We found that the benzenesulfonyl group in the 11-benzenesulfonyl indoloquinolines can be easily replaced by an alkylamino group as was exemplified by the reaction of the indoloquinoline 5cc with n-butylamine leading to 11-alkylamino derivative 7 (Scheme 6). The 11-(alkylamino)indolo[2,3-b]quinolines focused recently an interest as potential antimalarials.28
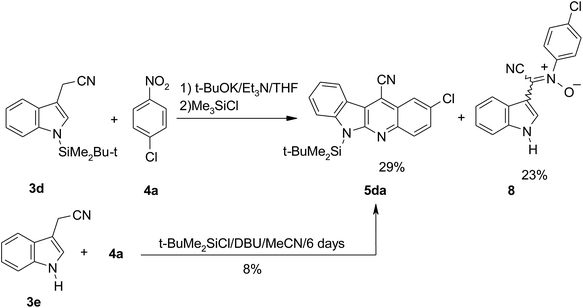
We attempted to use N-trialkylsilyl protected indole derivatives in these reactions, but our efforts were only partly successful. Attempts to obtain N-(trimethylsilyl)indol-3-ylacetonitrile from indol-3-ylacetonitrile under standard conditions using trimethylchlorosilane and amine (Et3N or DBU) failed. This indole derivative proved unstable and during isolation decomposed to the starting indol-3-ylacetonitrile. We found that more stable was N-(tert-butyldimethylsilyl)indol-3-ylacetonitrile 3d obtained from indol-3-ylacetonitrile and tert-butyldimethylchlorosilane. The anion of 3d generated with potassium tert-butoxide reacted with 4-chloronitrobenzene (4a) and the formed σH-adduct upon treatment with trimethylchlorosilane gave the expected 6-silylated derivative 5da in moderate yield 29% (Scheme 7). An additional product was isolated in this reaction. On the basis of the high resolution mass spectrum for this product molecular formula C16H10N3OCl was established, for which we proposed structure of nitrone 8, formed probably from direct attack of the carbanion of the indol-3-ylacetonitrile on the nitro group of the 4-chloronitrobenzene.
When we used DBU, a weaker base, the reaction of the silylated indol-3-ylacetonitrile 3d with 2,4-dichloronitrobenzene (4d) in the presence of additional tert-butyldimethylchlorosilane gave the expected 6-silylated tetracyclic derivative 5dd in 55% yield (Scheme 8).
 | ||
| Scheme 8 Reaction of N-silylated indol-3-ylacetonitrile with 4-chloronitrobenzene in the presence of weak base. | ||
Then, we attempted one-pot reaction of N–H unprotected indolyl-3-acetonitrile (3e) with nitrobenzenes. Thus when we mixed this indole derivative with 4-chloronitrobenzene with tert-butyldimethylchlorosilane and DBU in acetonitrile we observed by TLC slow formation of a product. After six-day stirring we isolated 6-silylated-2-chloro-11-cyanoindoloquinoline 5da in 8% yield (Scheme 7). Similarly, reactions of the nitrile 3e and ester 3f with more electrophilic 2,4-dichloronitrobenzene (4d) after three-weeks stirring at room temperature gave the expected products 5dd in 44% and 5fd in 33% yields, respectively (Scheme 9). Deprotection of 6 N-silylated derivatives can be executed by a simple treatment with tetrabutylammonium fluoride as exemplified by reaction of N-silylated derivative 5fd leading to indoloquinoline ester 9.
 | ||
| Scheme 9 Reaction of in situ generated N-silylated indol-3-ylmethyl derivatives with 2,4-dichloronitrobenzene. | ||
Conclusion
We developed simple synthesis of indolo[2,3-b]quinoline derivatives in reaction of nitroarenes with indol-3-ylmethyl carbanions generated from easily accessible starting materials. The elaborated method does not employ transition metals at any stage thus might be applicable in synthesis of pharmaceutically important intermediates.Experimental
Mp are uncorrected. 1H and 13C NMR spectra were recorded on a Bruker Avance 500 or Varian vnmr s500 (both 500 MHz for 1H and 125 MHz for 13C spectra) instruments at 298 K. Chemical shifts δ are expressed in parts per million (ppm) referred to TMS, coupling constants in hertz (Hz). Electron impact mass spectra (EI, 70 eV) were obtained on AutoSpec Premier spectrometer. Electrospray mass spectra (ESI) were obtained on 4000 Q-TRAP and SYNAPT G2-S HDMS. Elemental analyses were performed on Elementar Vario EL III instrument. Silica gel (Merck 60, 230–400 mesh) was used for column chromatography (CC). Toluene or hexane/ethyl acetate mixtures were used for elution. TLC analyses were performed on Merck Kieselgel 60 F254 Alufolien with hexane/ethyl acetate mixtures. All reagents and solvents were of reagent grade or purified according to standard methods before use. All reactions were run under argon atmosphere.Reactions of N-BOC protected indol-3-ylmethyl derivatives with nitroarenes. General procedure
To a stirred solution of indole derivative 3 (1 mmol), nitroarene 4 (1 mmol) and triethylamine (5 mmol) in THF (10 mL) cooled to −70 °C a solution of potassium tert-butoxide (0.14 g, 1.2 mmol) in THF (5 mL) was added maintaining the temperature below −65 °C. After 10 min to the reaction mixture trimethylchlorosilane (0.55 g, 5 mmol) was added dropwise maintaining the temperature below −65 °C. After addition the reaction mixture was stirred at this temperature for 3 h and then allowed to reach room temperature and stirred for 24 h. The reaction mixture was then quenched with aqueous saturated NH4Cl (20 mL). The precipitated product was separated, washed in water, dissolved in ethyl acetate and dried with Na2SO4. After evaporation of the solvent the product was subjected to column chromatography on silica gel with toluene as eluent.In the reactions of 3b with 4c and 3c with 4c the crude product was dissolved in methylene chloride (10 mL) and stirred with trifluoroacetic acid (2 mL) at room temperature for 24 h. The product was isolated by standard procedure.
The following compounds were obtained:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane, then AcOEt). Yellow solid, yield 25%: mp. 286.5–288 °C (AcOEt). IR (KBr) 3138, 3078, 2983, 1722, 1612, 1577, 1487, 1462, 1440, 1405, 1366, 1345, 1299, 1255, 1214 cm−1. 1H NMR (500 MHz, DMSO-d6) δ 1.47 (t, J = 7.1 Hz, 3H), 3.32 (s, 9H), 4.76 (q, J = 7.1 Hz, 2H), 7.32 (t, J = 7.4 Hz, 1H), 7.57 (d, J = 8.0 Hz, 1H), 7.64 (t, J = 7.6 Hz, 1H), 7.91 (dd, J = 9.0, 1.9 Hz, 1H), 8.03 (t, J = 7.8 Hz, 2H), 8.27 (d, J = 1.8, 1H), 12.15 (s, J = 8.4 Hz, 1H). 13C NMR (125 MHz; DMSO-d6) δ 13.97, 62.57, 111.47, 114.55, 116.41, 117.98, 120.29, 120.38, 123.28, 126.67, 129.45, 129.67, 129.71, 132.00, 142.35, 144.57, 152.57, 166.25 (one missing). HRMS (ESI) calcd for C18H14N2O2Br+: 369.0239; found: 369.0240.
hexane, then AcOEt). Yellow solid, yield 25%: mp. 286.5–288 °C (AcOEt). IR (KBr) 3138, 3078, 2983, 1722, 1612, 1577, 1487, 1462, 1440, 1405, 1366, 1345, 1299, 1255, 1214 cm−1. 1H NMR (500 MHz, DMSO-d6) δ 1.47 (t, J = 7.1 Hz, 3H), 3.32 (s, 9H), 4.76 (q, J = 7.1 Hz, 2H), 7.32 (t, J = 7.4 Hz, 1H), 7.57 (d, J = 8.0 Hz, 1H), 7.64 (t, J = 7.6 Hz, 1H), 7.91 (dd, J = 9.0, 1.9 Hz, 1H), 8.03 (t, J = 7.8 Hz, 2H), 8.27 (d, J = 1.8, 1H), 12.15 (s, J = 8.4 Hz, 1H). 13C NMR (125 MHz; DMSO-d6) δ 13.97, 62.57, 111.47, 114.55, 116.41, 117.98, 120.29, 120.38, 123.28, 126.67, 129.45, 129.67, 129.71, 132.00, 142.35, 144.57, 152.57, 166.25 (one missing). HRMS (ESI) calcd for C18H14N2O2Br+: 369.0239; found: 369.0240.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, then PhMe). Pale yellow solid, yield 11%: mp 142–144 °C (hexane
1, then PhMe). Pale yellow solid, yield 11%: mp 142–144 °C (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOEt). IR (KBr) 3092, 3054, 3002, 2976, 2958, 2931, 2904, 1937, 1733 (C
AcOEt). IR (KBr) 3092, 3054, 3002, 2976, 2958, 2931, 2904, 1937, 1733 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1721 (C
O), 1721 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1602, 1573, 1542, 1482, 1464, 1393, 1370, 1351, 1326, 1303, 1272, 1244, 1220, 1201 cm−1. 1H NMR (500 MHz; CDCl3) δ 1.55 (t, J = 7.2 Hz, 3H), 1.83 (s, 9H), 4.75 (q, J = 7.1 Hz, 2H), 7.41 (t, J = 7.6 Hz, 1H), 7.64 (t, J = 7.7 Hz, 1H), 7.89 (d, J = 2.0 Hz, 1H), 7.92 (d, J = 2.0 Hz, 1H), 7.96 (d, J = 7.8, 1H), 8.51 (d, J = 8.4 Hz, 1H). 13C NMR (125 MHz; CDCl3) δ 14.26, 28.45, 62.88, 84.87, 116.38, 117.14, 120.02, 122.76, 122.86, 122.91, 123.76, 130.08, 130.45, 130.69, 130.90, 134.61, 140.93, 141.18, 150.03, 151.08, 166.37. HRMS (ESI) calcd for C23H20N2O4Cl2: 458.0800; found: 458.0810.
O), 1602, 1573, 1542, 1482, 1464, 1393, 1370, 1351, 1326, 1303, 1272, 1244, 1220, 1201 cm−1. 1H NMR (500 MHz; CDCl3) δ 1.55 (t, J = 7.2 Hz, 3H), 1.83 (s, 9H), 4.75 (q, J = 7.1 Hz, 2H), 7.41 (t, J = 7.6 Hz, 1H), 7.64 (t, J = 7.7 Hz, 1H), 7.89 (d, J = 2.0 Hz, 1H), 7.92 (d, J = 2.0 Hz, 1H), 7.96 (d, J = 7.8, 1H), 8.51 (d, J = 8.4 Hz, 1H). 13C NMR (125 MHz; CDCl3) δ 14.26, 28.45, 62.88, 84.87, 116.38, 117.14, 120.02, 122.76, 122.86, 122.91, 123.76, 130.08, 130.45, 130.69, 130.90, 134.61, 140.93, 141.18, 150.03, 151.08, 166.37. HRMS (ESI) calcd for C23H20N2O4Cl2: 458.0800; found: 458.0810.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane). 1H NMR (500 MHz, DMSO-d6) δ 1.75 (s, 9H), 7.40–7.45 (m, 1H), 7.56–7.62 (m, 2H), 7.70 (dd, J = 7.4, 7.4 Hz, 1H), 7.74 (dd, J = 7.4, 7.4 Hz, 1H), 7.93 (dd, J = 9.0, 2.1 Hz, 1H), 7.94–7.99 (m, 2H), 8.19 (d, J = 9.0 Hz, 1H), 8.36 (d, J = 8.4 Hz, 1H), 8.90 (d, J = 8.3 Hz, 1H), 9.09 (d, J = 2.0 Hz, 1H). 13C NMR (125 MHz, DMSO-d6) δ 27.26, 84.67, 114.80, 118.72, 119.30, 121.42, 123.20, 123.41, 125.52, 128.62, 129.92, 130.42, 131.19, 131.49, 131.51, 134.41, 141.00, 141.02, 144.42, 148.57, 150.66. HRMS (ESI) m/z calcd for C26H22N2O4S35Cl+: 493.0989; found: 493.0990.
hexane). 1H NMR (500 MHz, DMSO-d6) δ 1.75 (s, 9H), 7.40–7.45 (m, 1H), 7.56–7.62 (m, 2H), 7.70 (dd, J = 7.4, 7.4 Hz, 1H), 7.74 (dd, J = 7.4, 7.4 Hz, 1H), 7.93 (dd, J = 9.0, 2.1 Hz, 1H), 7.94–7.99 (m, 2H), 8.19 (d, J = 9.0 Hz, 1H), 8.36 (d, J = 8.4 Hz, 1H), 8.90 (d, J = 8.3 Hz, 1H), 9.09 (d, J = 2.0 Hz, 1H). 13C NMR (125 MHz, DMSO-d6) δ 27.26, 84.67, 114.80, 118.72, 119.30, 121.42, 123.20, 123.41, 125.52, 128.62, 129.92, 130.42, 131.19, 131.49, 131.51, 134.41, 141.00, 141.02, 144.42, 148.57, 150.66. HRMS (ESI) m/z calcd for C26H22N2O4S35Cl+: 493.0989; found: 493.0990.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane). IR (KBr) 3489, 3378, 3306, 3086, 3002, 2987, 2932, 1950, 1909, 1870, 1795, 1756, 1738, 1729, 1631, 1592, 1550, 1541, 1481, 1457, 1443, 1428, 1397, 1371, 1353, 1307, 1248, 1204 cm−1. 1H NMR (500 MHz, CDCl3) δ 1.82 (s, 9H), 7.40–7.46 (m, 3H), 7.49 (t, J = 7.4 Hz, 1H), 7.68 (t, J = 7.9 Hz, 1H), 7.85–7.90 (m, 3H), 8.47 (d, J = 8.5 Hz, 1H), 9.15–9.19 (m, J = 9.0, 2.1 Hz, 2H). 13C NMR (125 MHz, CDCl3) δ 28.35, 85.38, 115.15, 119.15, 120.29, 122.75, 123.12, 123.76, 126.08, 129.43, 129.49, 130.31, 131.68, 131.71, 133.89, 134.48, 136.32, 141.39, 141.77, 141.94, 149.60, 151.00. HRMS (ESI) m/z calcd for C26H20N2O4NaS35Cl2+: 549.0419; found: 549.0414.
hexane). IR (KBr) 3489, 3378, 3306, 3086, 3002, 2987, 2932, 1950, 1909, 1870, 1795, 1756, 1738, 1729, 1631, 1592, 1550, 1541, 1481, 1457, 1443, 1428, 1397, 1371, 1353, 1307, 1248, 1204 cm−1. 1H NMR (500 MHz, CDCl3) δ 1.82 (s, 9H), 7.40–7.46 (m, 3H), 7.49 (t, J = 7.4 Hz, 1H), 7.68 (t, J = 7.9 Hz, 1H), 7.85–7.90 (m, 3H), 8.47 (d, J = 8.5 Hz, 1H), 9.15–9.19 (m, J = 9.0, 2.1 Hz, 2H). 13C NMR (125 MHz, CDCl3) δ 28.35, 85.38, 115.15, 119.15, 120.29, 122.75, 123.12, 123.76, 126.08, 129.43, 129.49, 130.31, 131.68, 131.71, 133.89, 134.48, 136.32, 141.39, 141.77, 141.94, 149.60, 151.00. HRMS (ESI) m/z calcd for C26H20N2O4NaS35Cl2+: 549.0419; found: 549.0414.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOEt). IR (KBr); 3051, 2952, 2926, 2854, 1916, 1878, 1729, 1622, 1596, 1562, 1478, 1459, 1391, 1373, 1341, 1311, 1287, 1267, 1254, 1243, 1200, 1154, 1116, 1097, 1067, 1029, 1007, 949 cm−1. 1H NMR (CDCl3) δ 0.90 (s, 6H), δ 1.07 (s, 9H), δ 1.55 (t, J = 7.2 Hz, 3H), δ 4.75 (q, J = 7.2 Hz, 2H), 7.28 (dd, J = 8.1, 7.4 Hz, 1H), 7.54 (dd, J = 8.4, 7.6 Hz, 1H), 7.68 (d, J = 8.4 Hz, 1H), 7.81 (d, J = 2.2 Hz, 1H), 7.95 (d, J = 2.2 Hz, 1H), 8.05 (d, J = 7.9 Hz, 1H). 13C NMR (CDCl3) δ −1.77, 14.31, 20.44, 26.79, 62.57, 114.15, 117.38, 120.79, 121.42, 121.50, 122.67, 123.58, 128.60, 129.11, 129.21, 129.73, 133.72, 140.81, 147.38, 158.04, 167.02. HRMS (ESI) m/z calcd for C24H27N2O2SiCl2+: 473.1219; found: 473.1213.
AcOEt). IR (KBr); 3051, 2952, 2926, 2854, 1916, 1878, 1729, 1622, 1596, 1562, 1478, 1459, 1391, 1373, 1341, 1311, 1287, 1267, 1254, 1243, 1200, 1154, 1116, 1097, 1067, 1029, 1007, 949 cm−1. 1H NMR (CDCl3) δ 0.90 (s, 6H), δ 1.07 (s, 9H), δ 1.55 (t, J = 7.2 Hz, 3H), δ 4.75 (q, J = 7.2 Hz, 2H), 7.28 (dd, J = 8.1, 7.4 Hz, 1H), 7.54 (dd, J = 8.4, 7.6 Hz, 1H), 7.68 (d, J = 8.4 Hz, 1H), 7.81 (d, J = 2.2 Hz, 1H), 7.95 (d, J = 2.2 Hz, 1H), 8.05 (d, J = 7.9 Hz, 1H). 13C NMR (CDCl3) δ −1.77, 14.31, 20.44, 26.79, 62.57, 114.15, 117.38, 120.79, 121.42, 121.50, 122.67, 123.58, 128.60, 129.11, 129.21, 129.73, 133.72, 140.81, 147.38, 158.04, 167.02. HRMS (ESI) m/z calcd for C24H27N2O2SiCl2+: 473.1219; found: 473.1213.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane 1
hexane 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, then 2
2, then 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yellow solid, yield 23%: mp. 231–234 °C (decomp.) (CH2Cl2
1). Yellow solid, yield 23%: mp. 231–234 °C (decomp.) (CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN). IR (KBr) 3175, 2919, 2857, 2221 (CN), 1722, 1615, 1584, 1615, 1584, 1517, 1487, 1475, 1435, 1402, 1371, 1325, 1276, 1242; cm−1. 1H NMR (500 MHz, DMSO-d6) δ 7.26–7.41 (m, 2H), 7.66 (d, J = 6.9 Hz, 2H), 7.74 (d, J = 6.6 Hz, 2H), 7.93 (d, J = 6.8 Hz, 2H), 8.23 (d, J = 6.8 Hz, 1H), 9.30 (s, 1H), 12.27 (s, 1H). 13C NMR (125 MHz, DMSO-d6) δ 104.47, 112.80, 115.07, 117.90, 118.60, 121.21, 123.30, 123.40, 126.29, 129.41, 130.68, 135.17, 136.05, 145.53. HRMS (ESI) m/z calcd for C16H10N3O35Cl+: 318.0410; found: 318.0407.
MeCN). IR (KBr) 3175, 2919, 2857, 2221 (CN), 1722, 1615, 1584, 1615, 1584, 1517, 1487, 1475, 1435, 1402, 1371, 1325, 1276, 1242; cm−1. 1H NMR (500 MHz, DMSO-d6) δ 7.26–7.41 (m, 2H), 7.66 (d, J = 6.9 Hz, 2H), 7.74 (d, J = 6.6 Hz, 2H), 7.93 (d, J = 6.8 Hz, 2H), 8.23 (d, J = 6.8 Hz, 1H), 9.30 (s, 1H), 12.27 (s, 1H). 13C NMR (125 MHz, DMSO-d6) δ 104.47, 112.80, 115.07, 117.90, 118.60, 121.21, 123.30, 123.40, 126.29, 129.41, 130.68, 135.17, 136.05, 145.53. HRMS (ESI) m/z calcd for C16H10N3O35Cl+: 318.0410; found: 318.0407.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1611, 1573, 1497, 1484, 1464, 1442, 1398, 1383, 1342, 1282, 1254, 1223, 1200 cm−1. 1H NMR (500 MHz, DMSO-d6) δ 1.49 (t, J = 7.1 Hz, 3H), 4.76 (q, J = 7.1 Hz, 2H), 7.32 (t, J = 7.6 Hz, 1H), 7.57 (d, J = 7.8 Hz, 1H), 7.64 (d, J = 7.6 Hz, 1H) 8.01 (d, J = 2.0 Hz, 1H), 8.05 (d, J = 7.8 Hz, 1H), 8.08 (dd, J = 2.0 Hz, 1H), 12.27 (s, 1H). 13C NMR (Bruker 125 MHz, DMSO-d6) δ 13.49, 62.25, 111.22, 115.22, 117.51, 120.15, 120.28, 122.55, 123.04, 126.79, 128.48, 129.56, 129.83, 131.79, 140.30, 142.34, 152.46, 165.52. HRMS (ESI) calcd for C18H13N2O235Cl2+: 359.0354; found: 359.0344.
O), 1611, 1573, 1497, 1484, 1464, 1442, 1398, 1383, 1342, 1282, 1254, 1223, 1200 cm−1. 1H NMR (500 MHz, DMSO-d6) δ 1.49 (t, J = 7.1 Hz, 3H), 4.76 (q, J = 7.1 Hz, 2H), 7.32 (t, J = 7.6 Hz, 1H), 7.57 (d, J = 7.8 Hz, 1H), 7.64 (d, J = 7.6 Hz, 1H) 8.01 (d, J = 2.0 Hz, 1H), 8.05 (d, J = 7.8 Hz, 1H), 8.08 (dd, J = 2.0 Hz, 1H), 12.27 (s, 1H). 13C NMR (Bruker 125 MHz, DMSO-d6) δ 13.49, 62.25, 111.22, 115.22, 117.51, 120.15, 120.28, 122.55, 123.04, 126.79, 128.48, 129.56, 129.83, 131.79, 140.30, 142.34, 152.46, 165.52. HRMS (ESI) calcd for C18H13N2O235Cl2+: 359.0354; found: 359.0344.Acknowledgements
This work was financially supported by National Scientific Center grant No 2012/07/B/ST5/00813.References
- C. Wright, Phytochem. Rev., 2005, 4, 55–61 CrossRef CAS.
- C. W. Wright, J. Pharm. Pharmacol., 2007, 59, 899–904 CrossRef CAS PubMed.
- L. Kaczmarek, W. Peczynska-Czoch, A. Opolski, J. Wietrzyk, E. Marcinkowska, J. Boratynski and J. Osiadacz, Anticancer Res., 1998, 18, 3133–3138 CAS.
- A. B. J. Bracca, D. A. Heredia, E. L. Larghi and T. S. Kaufman, Eur. J. Org. Chem., 2014, 7979–8003 CrossRef CAS.
- S. J. Holt and V. Petrow, J. Chem. Soc., 1948, 922–924 RSC.
- H. K. Kadam, P. T. Parvatkar and S. G. Tilve, Synthesis, 2012, 44, 1339–1342 CrossRef CAS.
- P. T. Parvatkar, P. S. Parameswaran and S. G. Tilve, J. Org. Chem., 2009, 74, 8369–8372 CrossRef CAS PubMed.
- T. Dhanabal, R. Sangeetha and P. S. Mohan, Tetrahedron, 2006, 62, 6258–6263 CrossRef CAS.
- Ł. Kaczmarek, R. Balicki, P. Nantka-Namirski, W. Peczyńska-Czoch and M. Mordarski, Arch. Pharm., 1988, 321, 463–467 CrossRef PubMed.
- B. Boganyi and J. Kaman, Tetrahedron, 2013, 69, 9512–9519 CrossRef CAS.
- P. T. Parvatkar, P. S. Parameswaran and S. G. Tilve, Tetrahedron Lett., 2007, 48, 7870–7872 CrossRef CAS.
- D. Basavaiah and D. Mallikarjuna Reddy, Org. Biomol. Chem., 2012, 10, 8774–8777 CAS.
- M. Mąkosza and K. Wojciechowski, Chem. Rev., 2004, 104, 2631–2666 CrossRef PubMed.
- M. Mąkosza and K. Wojciechowski, Heterocycles, 2014, 88, 75–101 CrossRef.
- M. Mąkosza and K. Wojciechowski, Top. Heterocycl. Chem., 2014, 37, 51–106 Search PubMed.
- M. Mąkosza and K. Wojciechowski, Chem. Heterocycl. Compd., 2015, 51, 210–222 CrossRef.
- K. Anczkiewicz, M. Królikiewicz, Z. Wróbel and K. Wojciechowski, Tetrahedron, 2015, 71, 3924–3931 CrossRef CAS.
- R. Bujok, A. Kwast, P. Cmoch and Z. Wróbel, Tetrahedron, 2010, 66, 698–708 CrossRef CAS.
- M. Bobin, A. Kwast and Z. Wróbel, Tetrahedron, 2007, 63, 11048–11054 CrossRef CAS.
- R. B. Davis and L. C. Pizzini, J. Org. Chem., 1960, 25, 1884–1888 CrossRef CAS.
- M. Więcław, M. Bobin, A. Kwast, R. Bujok, Z. Wróbel and K. Wojciechowski, Mol. Diversity, 2015, 19, 807–816 CrossRef PubMed.
- Z. Wróbel, Pol. J. Chem., 1998, 72, 2384–2388 Search PubMed.
- K. Wojciechowski, Eur. J. Org. Chem., 2001, 3587–3605 CrossRef CAS.
- V. H. M. Elferink and H. J. T. Bos, J. Chem. Soc., Chem. Commun., 1985, 882–883 RSC.
- A. D. Dilman, I. M. Lyapkalo, P. A. Belyakov, S. L. Ioffe, Y. A. Strelenko and V. A. Tartakovsky, Russ. Chem. Bull., 2000, 49, 1649–1650 CrossRef CAS.
- Z. Wróbel, Tetrahedron, 1998, 54, 2607–2618 CrossRef.
- Z. Wróbel, Eur. J. Org. Chem., 2000, 521–525 CrossRef.
- I. El Sayed, P. van der Veken, K. Steert, L. Dhooghe, S. Hostyn, G. van Baelen, G. Lemiere, B. U. W. Maes, P. Cos, L. Maes, J. Joossens, A. Haemers, L. Pieters and K. Augustyns, J. Med. Chem., 2009, 52, 2979–2988 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18626a |
| This journal is © The Royal Society of Chemistry 2015 |