Anisotropic swelling in hydrogels formed by cooperatively aligned megamolecules†
Abstract
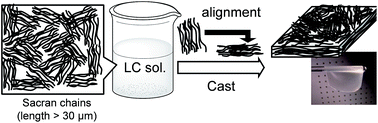
Sacran is a supergiant cyanobacterial polysaccharide with an extremely high absolute molecular weight that exceeds 107 g mol−1 (molecular length: over 30 μm). Sacran forms milli-scaled orientation domains in aqueous liquid crystalline (LC) state, even in trace concentrations i.e. 0.3 wt%. Aqueous sacran films that are cast from a LC state and annealed between 70–140 °C form self-standing sheets composed of oriented hydrogels. When sacran films swell, they experience changes in size that are 70 fold higher in relation to thickness than those that occur in relation to width. Either an increase in film thickness or a decrease in sacran chain length reduces swelling anisotropy, demonstrating that stress that occurs during drying can be effectively used to propagate the cooperative alignment of LC chains on a micrometer sized scale comparable with the thickness of self-standing films.

 Please wait while we load your content...
Please wait while we load your content...