Heat treatment of electrodeposited NiO films for improved catalytic water oxidation
Abstract
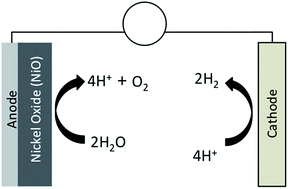
Recognizing the superior electrocatalytic properties of nickel oxide (NiO), we prepared cathodically electrodeposited nickel oxide (NiO) on fluorine doped tin oxide (FTO) glass substrates as binder free electrocatalysts for water oxidation. The electrodeposited nickel oxide film (NiO(ED)) showed remarkable improvement for electrocatalytic water oxidation after heat treatment. In particular, the NiO(ED)-400 catalyst (electrodeposited nickel oxide film heat treated at 400 °C) achieved appreciable current density ∼ 5 mA cm−2 for the oxygen evolution reaction (OER) at the overpotential of 0.45 V vs. RHE. These catalyst films were characterized for structural, morphological, thermal and electrochemical properties, where the results reveal that the dehydration during heat treatment permanently removes the structural water with a concomitant amorphous → crystalline transformation in NiO(ED) films, thereby making them more active catalysts for OER. In parallel investigations, nickel metal was electrodeposited on a stainless steel (SS) substrate, and was subsequently annealed in hot air to produce NiO(HA) films at different temperatures. The NiO(HA) films prepared by this method showed relatively high values of Tafel slopes and corresponding high overpotentials and low currents for OER, when compared to the NiO(ED) films. Hence, simple heat treatment of the cathodically electrodeposited nickel oxide (NiO(ED)) films showed remarkable improvements in their catalytic performances for oxygen evolution reaction, thereby making them efficient electrocatalysts for water oxidation.

 Please wait while we load your content...
Please wait while we load your content...