Kinetics of hydrazine reduction of thin films of graphene oxide and the determination of activation energy by the measurement of electrical conductivity
Seo Gyun Kima,
Soon Sik Leea,
Eunsu Leeb,
Jinhwan Yoon*b and
Heon Sang Lee*a
aDepartment of Chemical Engineering, Dong-A University, 37 Nakdong-daero, 550 beon-gil, Saha-gu, Busan 49315, Korea. E-mail: heonlee@dau.ac.kr
bDepartment of Chemistry, Dong-A University, 37 Nakdong-daero, 550 beon-gil, Saha-gu, Busan 49315, Korea. E-mail: jyoon@dau.ac.kr
First published on 25th November 2015
Abstract
We fabricated transparent conductive gas barrier films by reducing graphene oxide (GO) coatings on polyethylene terephthalate (PET) films. GO solutions were cast on pure PET films, and the film surfaces were chemically reduced in a hydrazine hydrate solution. By measurement of the electrical conductivity of the films, the chemical reduction kinetics at the surface were examined as a function of reduction time, reductant concentration, and temperature. Based on these measurements, we determined the activation energy, 16 kcal mol−1, which corresponds to the barrier for the anchoring of the hydrazine either opposite to an epoxide ring or at a top site adjacent to a surface OH group. When the GO film was reduced to a carbon-to-oxygen (C/O) atomic ratio of 4.7, the electrical conductivity was 2.0 × 103 S m−1, with a light transmittance of 67% at 550 nm. We also revealed that the oxygen permeability of the film is a single exponential function of C/O atomic ratio. The reduced GO (rGO)/PET films exhibited an oxygen permeability of 7.97 × 10−8 Barrer (1 Barrer = 10−10 cm3 cm cm−2 s−1 cm Hg−1), providing optically transparent and electrically conductive polymer films for gas barrier applications.
1. Introduction
Graphene, which comprises unique single layers of sp2-bonded carbon atoms, exhibits remarkable thermal, mechanical, electrical,1–4 and physical properties, including impermeability to standard gases.5–8 The unique properties of graphene sheets hold great promise for a variety of applications, including polymer composites,7–9 transparent electrodes,10,11 energy storage,12,13 gas sensors,14 and field effect transistors.15,16 Many researchers have developed methods to prepare graphene sheets, such as mechanical exfoliation (“Scotch-tape method”),16 chemical vapor deposition,17 epitaxial growth,18 liquid-phase exfoliation,19,20 and chemical oxidation to dispersed graphene oxide (GO).21 The latter approach can be conducted on large scale, based on the Brodie,1 Staudenmaier,22 and Hummers' methods.7,23,24In GO, oxygen-containing functionalities such as alcohol and epoxide groups are situated on the basal planes, and carboxyl and ketone groups are positioned at the edges of the sheets. These functional groups impart hydrophilicity and dispersibility to the GO in aqueous solution.25–27 GO is an insulating material due to these oxygen groups installed at its sp3-hybridized carbons, which disrupt electrical conduction pathways.28 However, the electrical properties of GO can be recovered through reduction. Several groups have reported the chemical reduction of GO by reducing agents such as hydrazine,28,29 sodium borohydrazine,30 dimethylhydrazine,31 hydrogen sulfide,32 hydroquinone,33 and aluminum powder.34 GO can also be reduced by exposure to UV35 and thermal energy.29,36 As it is reduced in aqueous solution, the exfoliated GO can be easily restacked.37 Surfactants and polymers have been used as effective stabilizers to prevent aggregation during reduction.31,38 Although stabilizers prevent reaggregation, their effects are limited. For example, the electrical conductivities of a films using sodium dodecyl sulfate (SDS)-dispersed graphene39 or carbon nanotubes (CNTs)40 were lower than that based on pristine graphene or CNT.39,40 It was reported that the removal of the remaining SDS increased the cross-junction resistance between the CNT networks and enhanced the metallicity of the CNT.40
To avoid the restacking problem, another strategy would be the fabrication of a GO film, which may not be electrically conductive prior to its reduction. Then, electrical conductivity could be achieved by surface chemical reduction of the GO film. Here, we pursed to reduction of GO coated on polyethylene terephthalate (PET) films by aqueous hydrazine hydrate.
Motivated by the enhanced electrical conductivity during reduction of GO, we attempted to monitor the extent of the reduction by measurement of the electrical conductivity of GO coated on a polymer film during the reaction. These measurements enable the prompt determination for the surface chemical reduction of GO on polymer film. Subsequently, based on the measurements of the electrical conductivity under various reduction conditions, we examined the reduction kinetics by varying the reduction time, reductant concentration, and temperature.
Regarding potential application of rGO coated on PET film prepared by developed method, we envisioned a conductive transparent oxygen barrier film. According to the previous studies, polymer films containing GO or rGO showed gas barrier property due to the impermeable property of the graphene monolayer.7,41,42 Recent study revealed that the reduction of GO enhanced the barrier property of the film,41 however, quantitative investigation for relation between degree of reduction and gas permeability has not been reported. Here, we investigated the conductivity, oxygen permeability and light transmittance by varying the carbon-to-oxygen (C/O) atomic ratio of GO through surface chemical reduction.
2. Experimental
2.1 Preparation of reduced graphene oxide (rGO) films
PET films with a thickness of 23 μm were provided by SKC Co., Ltd (Seoul, Korea). GO solution was prepared from flake graphite (4 μm nominal particle size, Qingdao Kropfmuehl Graphite Co., Ltd.) by the modified Hummers' method.7,23,24 Isopropyl alcohol (IPA) was added in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio to aqueous GO and the suspension was sonicated for 1 h. The GO/water/IPA dispersion was cast onto a PET film using a bar coater with a rate of 20 mm s−1 at RT and then dried at 100 °C for 10 min. The bar coater surface is coated by metal wire with diameter of 50 μm. The resulting films were dried again in a vacuum oven at 100 °C for 1 h. The reduction of the GO coatings on the PET films was carried out upon their immersion into hydrazine hydrate solution under various conditions (e.g., hydrazine concentration, temperature, and reduction time). The rGO films were washed twice with distilled water for 5 min, and then dried in a vacuum oven at 100 °C for 2 h.
1 volume ratio to aqueous GO and the suspension was sonicated for 1 h. The GO/water/IPA dispersion was cast onto a PET film using a bar coater with a rate of 20 mm s−1 at RT and then dried at 100 °C for 10 min. The bar coater surface is coated by metal wire with diameter of 50 μm. The resulting films were dried again in a vacuum oven at 100 °C for 1 h. The reduction of the GO coatings on the PET films was carried out upon their immersion into hydrazine hydrate solution under various conditions (e.g., hydrazine concentration, temperature, and reduction time). The rGO films were washed twice with distilled water for 5 min, and then dried in a vacuum oven at 100 °C for 2 h.
2.2 Characterization
The morphology of the graphite powder was observed by field-emission scanning electron microscopy (FE-SEM, JSM-6700F) after gold sputtering. A high-resolution transmission electron microscopy (HRTEM) images of the rGO films were obtained with a JEM-2010 HRTEM instrument. The samples were dropped onto lacey carbon-coated 400 mesh copper grids and dried in air before TEM imaging. The electrical conductivities of the GO and rGO samples were measured with a Keithley 2400 electrometer, using the two-point probe technique. Fourier-transform infrared (FT-IR) spectra were recorded with a Nicolet 380 spectrometer to study the degree of GO reduction. Air-dried GO and rGO films were abraded with KBr and pressed to prepare pellets. X-ray photoelectron spectroscopy (XPS) measurements were performed with a MultiLab 2000 (Thermo Scientific, UK) using Mg Kα radiation (hν = 1253.68 eV, 300 W). The light transmittance of samples was measured with a UV-vis spectrophotometer (Cary 5000) at 550 nm. The oxygen permeabilities of 10 × 10 cm2 of GO and rGO film samples were measured with an OX-TRAN Model 2/21(MOCON) oxygen permeation analyzer at 23 °C and 0% relative humidity.3. Results and discussion
The GO-coated PET films were fabricated by the bar-coating of GO solution onto PET substrates, which is a simple method amenable to mass production. In this process, the thickness of the GO can be easily controlled by adjusting the solution concentration. The reduction of GO on the PET films was carried out by immersing the films into hydrazine hydrate solution while varying the temperature and hydrazine concentration. To monitor the extent of the reduction, we measured the electrical conductivity of the GO-coated PET films using the two-point probe method during the reduction reaction. Since the electrical conductivity of the rGO is restored as a consequence of the recovered π-conjugated network structure, its measurement can be a direct criterion by which to judge the chemical structural change of GO due to the reduction of oxygen-containing groups. In this work, the extent of the reduction reaction could be followed in situ by monitoring the electrical conductivity values of the samples.The effects of the reduction temperature and concentration of the reductant on the electrical conductivity of the rGO films are presented in Fig. 1. The electrical conductivities of the GO-coated PET films were dependent on the reduction time, concentration, and medium temperature. For the GO-coated PET film in 10% hydrazine hydrate solution at RT, the electrical conductivity increases with reduction time. As mentioned above, the increase in the electrical conductivity results from the reduction of the GO film surface. Similar electrical conductivity variations were observed for other films at different hydrazine concentrations at the same temperature; the electrical conductivity rapidly increased as the hydrazine concentration increased. At a treatment time of 120 s, we found that the electrical conductivity values for the films were proportional to the hydrazine solution concentration, indicating that the reduction rate increases with the reductant concentration.
As shown in Fig. 1b, the electrical conductivity also rapidly increases with temperature. For the film in 35% hydrazine hydrate solution at RT, the electrical conductivity reaches a saturated value of ∼103 S m−1 in ∼6000 s; the same film at 90 °C exhibits an electrical conductivity of ∼103 S m−1 in 120 s. These saturated electrical conductivity values (103 S m−1) are comparable to those in previous reports.28,43 We also found that the electric conductivity values for the films are proportional to the temperature of the hydrazine hydrate solution, indicating that the reduction rate increases with the medium temperature.
To the best of our knowledge, this is the first report of real-time electrical conductivity measurements of GO coated on a polymer film during chemical reduction by hydrazine as a method to monitor the extent of the reduction. The measured data clearly show a consistent improvement in GO film conductivity with reduction. These results provide important information regarding the reduction of the GO-coated PET films. First, the electrical conductivity of the films can be customized by adjusting the time, concentration, and temperature of the treatment. Since the electrical conductivity of the GO-coated PET film can be enhanced by the removal of oxygen groups from the GO, the average electrical conductivities of GO films treated with hydrazine ranged from 3.5 × 10−3 to 2.0 × 103 S m−1 depending on the extent of reduction. Second, the reduction kinetics for the GO coated on the PET with hydrazine solution is more sensitive to the medium temperature than the concentration of the solution. Compared to the conductivity changes as a function of the hydrazine solution concentration at constant temperature, the reduction kinetics showed a greater rate of change with increases in temperature at the same concentration, as shown in Fig. 1.
To confirm that the increases in the electrical conductivities of the GO-coated PET films are due to the reduction of GO, we obtained the FT-IR and XPS spectra of a sample treated in aqueous hydrazine hydrate (35 wt%) at RT for 720 min. Fig. 2 shows the FT-IR spectra for the GO before and after hydrazine treatment. The presence of oxygen functionalities in the GO sheets is confirmed by the characteristic vibrations at 3400 cm−1 (O–H stretching vibrations), 1721 cm−1 (stretching vibrations from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597 cm−1 (skeletal vibrations from unoxidized graphene domains), 1400 cm−1 (O–H deformation), 1220 cm−1 (C–OH stretching vibrations), and 1054 cm−1 (C–O stretching peak). The spectrum of the sample after hydrazine treatment revealed the removal or significant reduction of these characteristic vibrations due to oxygenated functional groups. Thus, the oxygen functional groups on the GO were removed by the reduction reaction in the hydrazine solution.
O), 1597 cm−1 (skeletal vibrations from unoxidized graphene domains), 1400 cm−1 (O–H deformation), 1220 cm−1 (C–OH stretching vibrations), and 1054 cm−1 (C–O stretching peak). The spectrum of the sample after hydrazine treatment revealed the removal or significant reduction of these characteristic vibrations due to oxygenated functional groups. Thus, the oxygen functional groups on the GO were removed by the reduction reaction in the hydrazine solution.
 | ||
| Fig. 2 FT-IR spectra for GO and rGO after treatment in 35 wt% N2H4·H2O solution at RT for 720 min (Tr = 720 min). | ||
However, the peaks at 1721 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration) and 1112 cm−1 (C–O stretching peak) are still observed after reduction. These correspond to the carboxyl functional groups of GO, and indicate their retention even after reduction in hydrazine solution for 720 min. The presence of the carboxyl groups contributes to the charge of the rGO surface post-reduction.44,45 The peaks at 3400 and 1400 cm−1 are significantly reduced but still remain, indicating that the hydroxyl groups of GO are not entirely removed.
O stretching vibration) and 1112 cm−1 (C–O stretching peak) are still observed after reduction. These correspond to the carboxyl functional groups of GO, and indicate their retention even after reduction in hydrazine solution for 720 min. The presence of the carboxyl groups contributes to the charge of the rGO surface post-reduction.44,45 The peaks at 3400 and 1400 cm−1 are significantly reduced but still remain, indicating that the hydroxyl groups of GO are not entirely removed.
Fig. 3 shows the XPS spectra for the GO-coated PET films before and after hydrazine treatment. In Fig. 3a, the deconvolution of the C 1s peak of GO indicates the existence of two main carbon bond components arising from C–C (284.89 eV) and C–O (286.92 eV) groups, and two minor components from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.73 eV) and O–C
O (288.73 eV) and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (290.61 eV) groups.38,44 As summarized in Table 1, most of the oxygenated functional groups in GO are epoxide and hydroxyl groups. After the hydrazine treatment of GO for 720 min, the intensities of two carbon bonding motifs were decreased (C–O (286.61 eV) and C
O (290.61 eV) groups.38,44 As summarized in Table 1, most of the oxygenated functional groups in GO are epoxide and hydroxyl groups. After the hydrazine treatment of GO for 720 min, the intensities of two carbon bonding motifs were decreased (C–O (286.61 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.38 eV)), whereas the intensities of the two peaks at 285.03 and 290.23 eV, which respectively correspond to non-oxygenated C and O–C
O (288.38 eV)), whereas the intensities of the two peaks at 285.03 and 290.23 eV, which respectively correspond to non-oxygenated C and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, were slightly increased. We found that the integrations from the oxygen-functionalized carbon peaks for the hydrazine-treated sample were much smaller than those in GO. Epoxides on the GO surface are easily reduced during hydrazine treatment. Even though some carboxyl groups attached to GO remain after reduction, these groups have only limited influence on the conductivity of the rGO. XPS analysis reveals that the GO coated on the PET film was reduced to rGO after hydrazine treatment for 720 min, which is consistent with the FT-IR spectra.
O bonds, were slightly increased. We found that the integrations from the oxygen-functionalized carbon peaks for the hydrazine-treated sample were much smaller than those in GO. Epoxides on the GO surface are easily reduced during hydrazine treatment. Even though some carboxyl groups attached to GO remain after reduction, these groups have only limited influence on the conductivity of the rGO. XPS analysis reveals that the GO coated on the PET film was reduced to rGO after hydrazine treatment for 720 min, which is consistent with the FT-IR spectra.
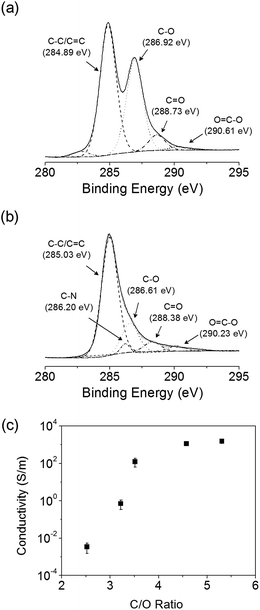 | ||
| Fig. 3 C 1s XPS spectra for (a) GO and (b) rGO treated in 35 wt% N2H4·H2O at RT for 720 min. (c) Electrical conductivity of rGO films as a function of carbon-to-oxygen (C/O) atomic ratio. | ||
| Fitting of the C 1s peak binding energy [eV] (relative atomic percentage [%]) | |||||
|---|---|---|---|---|---|
C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
C–N | C–O | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O C–O |
|
| GO | 284.86 (53.76) | — | 286.92 (38.31) | 288.73 (6.45) | 290.61 (1.48) |
| rGO (Tr = 720 min) | 285.03 (72.62) | 286.20 (3.45) | 286.61 (11.88) | 288.38 (8.54) | 290.23 (3.49) |
From the XPS analysis, we further calculated the carbon-to-oxygen (C/O) atomic ratio on the GO surface as a function of treatment time, and then investigated the correlation between the electrical conductivity and this ratio (Fig. 3c). GO exhibits a C/O atomic ratio of 2.5 and electrical conductivity of 3.5 × 10−2 S m−1. The electrical conductivity of the GO films is enhanced by the increase of the C/O atomic ratio, which is caused by reduction. When the C/O atomic ratio is ∼4.7, the electrical conductivity reaches a saturation point of ∼103 S m−1. As the reduction proceeds (i.e., the C/O atomic ratio increases), the restored π-conjugated network structure enhances the electrical conductivity of the film.
We attempted to determine the activation energy for the reduction of GO using the conductivity values depending on the treatment times at various hydrazine concentrations and temperatures. The oxygen conversion (![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) o) is defined as the surface atomic ratio in GO to that in rGO, which is determined by XPS analysis. The oxygen conversion was monitored by in situ measurement of the electrical conductivity of the films, using the calibration curve in Fig. 3c. The effect of hydrazine hydrate concentration on the oxygen conversion of GO at RT is presented in Fig. 4a, and the effect of temperature on the conversion in 10% hydrazine hydrate is shown in Fig. 4b. The initial reaction rate (RA) was determined from the data in Fig. 4a and b as follows.
o) is defined as the surface atomic ratio in GO to that in rGO, which is determined by XPS analysis. The oxygen conversion was monitored by in situ measurement of the electrical conductivity of the films, using the calibration curve in Fig. 3c. The effect of hydrazine hydrate concentration on the oxygen conversion of GO at RT is presented in Fig. 4a, and the effect of temperature on the conversion in 10% hydrazine hydrate is shown in Fig. 4b. The initial reaction rate (RA) was determined from the data in Fig. 4a and b as follows.
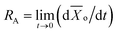 | (1) |
The effects of temperature and hydrazine hydrate concentration on the initial reaction rate are presented in Fig. 4c and d, respectively. Since the reaction rate depends on the concentrations of reactant and product as well as the temperature, as reported elsewhere,46 the initial reaction rate for an arbitrary reaction mechanism can be expressed as follows:
RA = f(φ)k![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(ΔE/RT) exp(ΔE/RT)
| (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K−1
K−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol−1), and T is the temperature. The activation energy for the initial reaction rate in Fig. 4c is determined to be 16.87 kcal mol−1, as seen in Fig. 5.
mol−1), and T is the temperature. The activation energy for the initial reaction rate in Fig. 4c is determined to be 16.87 kcal mol−1, as seen in Fig. 5.
 | ||
| Fig. 5 Activation energy for the reduction of GO calculated from the reaction rate depending on the temperature. | ||
Here, two kinetic processes may be considered. One is epoxide ring opening and the other is OH hydrogenation. The barrier for epoxide ring opening was calculated by Kim and coworkers and reported as 10.2 kcal mol−1 for the direct Eley–Rideal case.47 The theoretical value of the barrier for OH hydrogenation was reported to be 6.7 kcal mol−1.47 Therefore, the experimentally determined activation energy in Fig. 4c might be closer to the barrier for epoxide ring opening than that for OH hydrogenation. However, the barrier for the anchoring of NHNH2 at an on-top site adjacent to a surface OH group was reported to be 10.6 kcal mol−1.47 Therefore, the barriers for the epoxide ring opening and OH hydrogenation may be similar macroscopically. The barrier for the anchoring of NH2NH2 to the opposite side of the epoxide ring was calculated by Gao and coworkers as 15–25 kcal mol−1.29 Thus, the experimentally determined activation energy, 16 kcal mol−1, may correspond to the barrier for the anchoring of the hydrazine either opposite to an epoxide ring or at a top site adjacent to a surface OH group. Thus, we conclude that the activation energy determined in this work reflects the overall reduction reactions of the epoxide and hydroxyl groups on the GO surface.
Fig. 6a shows photographs of the GO-coated PET films before and after reduction, respectively. Both films are transparent, and the light brown GO film was changed to a pale grey after chemical reduction of the surface.
 | ||
| Fig. 6 (a) Optical images of the GO (upper)- and rGO (lower)-coated PET films. (b) Measured light transmittance at 550 nm of films as a function of C/O atomic ratio. | ||
We measured the light transmittance at 550 nm for rGO films as a function of the C/O atomic ratios obtained via XPS analysis. As seen in Fig. 6b, the transmittance was decreased by the increase of the C/O atomic ratio. Since the enhancement of the electrical conductivity of the rGO due to the restoration of the sp2 π-conjugated network increases the charge-carrier concentration and mobility, the reduction of GO improves its reflection of incident light. Therefore, the transmittance of the films decreases as the C/O atomic ratio increases because of GO reduction. Thus, we can successfully fabricate a transparent electrically conductive film that exhibits an electrical conductivity of 2.0 × 103 S m−1 with a light transmittance of 67% at 550 nm.
The oxygen permeability (P) of the rGO-coated PET films was measured by oxygen permeation analyzer. The P of the rGO layer can be estimated using eqn (3), since the permeability of the rGO-coated film is determined by the harmonic mean of the permeabilities of the rGO and PET layers.
 | (3) |
Fig. 7 shows the P values for the rGO-coated PET film and rGO layer. The oxygen permeability of the rGO/PET film at C/O ratio of 4.47 is 3.34 × 10−4 Barrer (1 Barrer = 10−10 cm3 cm cm−2 s−1 cm Hg−1). This permeability is comparable with the reported values of 4.58 × 10−4 Barrer for rGO/EVOH/PET film42 and 1.51 × 10−4 Barrer for rGO/PVA/PET film.7 For the rGO layer with a C/O atomic ratio of 4.47, P is 7.97 × 10−8 Barrer which is 3 × 105 times lower than that of the pure PET film, indicating that rGO seems to act as a good oxygen barrier layer. The P for the rGO is exponentially decreased by increasing the C/O atomic ratio, as seen in Fig. 7. That is, the oxygen permeability of the film is a single exponential function of C/O atomic ratio.
One of the reasons for decreasing oxygen permeability is short interlayer distance of the rGO. The interlayer spacing of rGO is far smaller than that of GO,7 which plays a crucial role to control gas flux through the GO or rGO films. Since the molecular size of oxygen is comparable to the interlayer spacing of the rGO stack, oxygen permeability of the film decrease with increase of C/O atomic ratio. Furthermore, owing to the bumpy structure of rGO sheets,7 tortuous diffusion path may be formed in rGO layer on PET film. This leads to the drastic reduction of apparent diffusivity though the rGO-coated film. The oxygen flux can be expressed as Smoluchowski equation as following.
 | (4) |
Therefore, the exponential decrease of oxygen flux can be attributed to the increase of friction factor (ζ) and/or to the decrease of chemical potential gradient (∂μ/∂x). In oxygen diffusion though the tortuous path in rGO-layer, the friction factor may not be decreased exponentially with the increase of C/O atomic ratio. So, the exponential decrease of oxygen permeability seems mainly ascribed to the decrease of chemical potential gradient for high C/O atomic ratio sample. For this case, the chemical potential difference (Δμ) can be approximated as following.
 | (5) |
Good water-vapor-barrier properties have also been reported for rGO in organic photovoltaic devices.50 However, it should be noted that the gas/vapor flux through the rGO layer does not decrease linearly with the increase in thickness, since the rGO layer cannot be treated as homogeneous material in the normal direction to its surface. The decrease in the gas flux with the increasing thickness of the rGO layer should be much smaller than that expected for a homogeneous material.
4. Conclusion
By the chemical reduction of GO coated on a polymer film, we could fabricate transparent electrically conductive films that show enhanced oxygen barrier properties. The reduction of GO could be achieved in hydrazine hydrate solution, and the extent of the structural changes as a function of the reaction conditions could be monitored by measuring the electrical conductivity during hydrazine treatment. Using these electrical conductivity measurements, the kinetics for the surface chemical reduction was examined for various reduction times, reductant concentrations, and temperatures. We determined the reaction rate for the reduction of GO, and an activation energy of 16.87 kcal mol−1 was obtained. We also revealed that the oxygen permeability of the film is a single exponential function of C/O atomic ratio. For an rGO layer with a C/O atomic ratio of 4.47, P is 7.97 × 10−8 Barrer. We believe that this information will be useful in fabricating transparent, electrically conductive films with gas barrier properties based on GO and polymer films.Acknowledgements
This work was supported by the Graphene Materials/Components Development Project (10044366) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea), and the Basic Research (2014R1A1A2054466) by the Ministry of Education (MOE) and the National Research Foundation of Korea (NRF).References
- B. C. Brodie, Philos. Trans. R. Soc. London, 1859, 249–259 CrossRef.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nature, 2007, 448, 457–460 CrossRef CAS PubMed.
- J. S. Bunch, S. S. Verbridge, J. S. Alden, A. M. van der Zande, J. M. Parpia, H. G. Craighead and P. L. McEuen, Nano Lett., 2008, 8, 2458–2462 CrossRef CAS PubMed.
- R. Nair, H. Wu, P. Jayaram, I. Grigorieva and A. Geim, Science, 2012, 335, 442–444 CrossRef CAS PubMed.
- H. M. Kim, J. K. Lee and H. S. Lee, Thin Solid Films, 2011, 519, 7766–7771 CrossRef CAS.
- H. Kim, Y. Miura and C. W. Macosko, Chem. Mater., 2010, 22, 3441–3450 CrossRef CAS.
- H. Kim, A. A. Abdala and C. W. Macosko, Macromolecules, 2010, 43, 6515–6530 CrossRef CAS.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270–274 CrossRef CAS PubMed.
- X. Wang, L. Zhi and K. Müllen, Nano Lett., 2008, 8, 323–327 CrossRef CAS PubMed.
- C. Xu, B. Xu, Y. Gu, Z. Xiong, J. Sun and X. Zhao, Energy Environ. Sci., 2013, 6, 1388–1414 CAS.
- Y. Qian, A. Vu, W. Smyrl and A. Stein, J. Electrochem. Soc., 2012, 159, A1135–A1140 CrossRef CAS.
- F. Schedin, A. Geim, S. Morozov, E. Hill, P. Blake, M. Katsnelson and K. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- M. Jin, H.-K. Jeong, W. J. Yu, D. J. Bae, B. R. Kang and Y. H. Lee, J. Phys. D: Appl. Phys., 2009, 42, 135109 CrossRef.
- K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, Y. Zhang, S. Dubonos, I. Grigorieva and A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- A. N. Obraztsov, Nat. Nanotechnol., 2009, 4, 212–213 CrossRef CAS PubMed.
- C. Berger, Z. Song, X. Li, X. Wu, N. Brown, C. Naud, D. Mayou, T. Li, J. Hass, A. N. Marchenkov, E. H. Conrad, P. N. First and W. A. de Heer, Science, 2006, 312, 1191–1196 CrossRef CAS PubMed.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. McGovern, B. Holland, M. Byrne, Y. K. Gun'Ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563–568 CrossRef CAS PubMed.
- C.-J. Shih, A. Vijayaraghavan, R. Krishnan, R. Sharma, J.-H. Han, M.-H. Ham, Z. Jin, S. Lin, G. L. Paulus, N. F. Reuel, Q. H. Wang, B. Daniel and M. S. Strano, Nat. Nanotechnol., 2011, 6, 439–445 CrossRef CAS PubMed.
- S. Park and R. S. Ruoff, Nat. Nanotechnol., 2009, 4, 217–224 CrossRef CAS PubMed.
- L. Staudenmaier, Ber. Dtsch. Chem. Ges., 1898, 31, 1481–1487 CrossRef CAS.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- N. I. Kovtyukhova, P. J. Ollivier, B. R. Martin, T. E. Mallouk, S. A. Chizhik, E. V. Buzaneva and A. D. Gorchinskiy, Chem. Mater., 1999, 11, 771–778 CrossRef CAS.
- H. He, J. Klinowski, M. Forster and A. Lerf, Chem. Phys. Lett., 1998, 287, 53–56 CrossRef CAS.
- D. W. Boukhvalov and M. I. Katsnelson, J. Am. Chem. Soc., 2008, 130, 10697–10701 CrossRef CAS PubMed.
- N. R. Wilson, P. A. Pandey, R. Beanland, R. J. Young, I. A. Kinloch, L. Gong, Z. Liu, K. Suenaga, J. P. Rourke, S. J. York and J. Sloan, ACS Nano, 2009, 3, 2547–2556 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- X. Gao, J. Jang and S. Nagase, J. Phys. Chem. C, 2009, 114, 832–842 Search PubMed.
- Y. Si and E. T. Samulski, Nano Lett., 2008, 8, 1679–1682 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, G. H. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS PubMed.
- U. Hofmann and A. Frenzel, Kolloid-Z., 1934, 68, 149–151 CrossRef CAS.
- G. Wang, J. Yang, J. Park, X. Gou, B. Wang, H. Liu and J. Yao, J. Phys. Chem. C, 2008, 112, 8192–8195 CAS.
- Z. Fan, K. Wang, T. Wei, J. Yan, L. Song and B. Shao, Carbon, 2010, 48, 1686–1689 CrossRef CAS.
- G. Williams, B. Seger and P. V. Kamat, ACS Nano, 2008, 2, 1487–1491 CrossRef CAS PubMed.
- M. J. McAllister, J.-L. Li, D. H. Adamson, H. C. Schniepp, A. A. Abdala, J. Liu, M. Herrera-Alonso, D. L. Milius, R. Car, R. K. Prud'homme and I. A. Aksay, Chem. Mater., 2007, 19, 4396–4404 CrossRef CAS.
- J. Liu, Y. Wang, S. Xu and D. D. Sun, Mater. Lett., 2010, 64, 2236–2239 CrossRef CAS.
- S. Stankovich, R. D. Piner, X. Chen, N. Wu, S. T. Nguyen and R. S. Ruoff, J. Mater. Chem., 2006, 16, 155–158 RSC.
- M. Lotya, Y. Hernandez, P. J. King, R. J. Smith, V. Nicolosi, L. S. Karlsson, F. M. Blighe, S. De, Z. Wang and I. McGovern, J. Am. Chem. Soc., 2009, 131, 3611–3620 CrossRef CAS PubMed.
- H.-Z. Geng, K. K. Kim, K. P. So, Y. S. Lee, Y. Chang and Y. H. Lee, J. Am. Chem. Soc., 2007, 129, 7758–7759 CrossRef CAS PubMed.
- Y. Su, V. Kravets, S. Wong, J. Waters, A. Geim and R. Nair, Nat. Commun., 2014, 5, 4843 CrossRef CAS PubMed.
- H. M. Kim and H. S. Lee, Carbon Lett., 2014, 15, 50–56 CrossRef.
- S. Pei and H.-M. Cheng, Carbon, 2012, 50, 3210–3228 CrossRef CAS.
- Y. Xu, H. Bai, G. Lu, C. Li and G. Shi, J. Am. Chem. Soc., 2008, 130, 5856–5857 CrossRef CAS PubMed.
- D. Li, M. B. Müller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- O. Levenspiel, Chemical reaction engineering, Jone Wiley & Sons, New York, 2nd edn, 1972 Search PubMed.
- M. C. Kim, G. S. Hwang and R. S. Ruoff, J. Chem. Phys., 2009, 131, 064704 CrossRef PubMed.
- R. Nair, P. Blake, A. Grigorenko, K. Novoselov, T. Booth, T. Stauber, N. Peres and A. Geim, Science, 2008, 320, 1308 CrossRef CAS PubMed.
- X. Zhou, X. Huang, X. Qi, S. Wu, C. Xue, F. Y. Boey, Q. Yan, P. Chen and H. Zhang, J. Phys. Chem. C, 2009, 113, 10842–10846 CAS.
- T. Kim, J. Kang, S. Yang, S. Sung, Y. Kim and C. Park, Energy Environ. Sci., 2014, 7, 3403–3411 CAS.
| This journal is © The Royal Society of Chemistry 2015 |



