Zinc titanium glycolate acetate hydrate and its transformation to zinc titanate microrods: synthesis, characterization and photocatalytic properties†
Abstract
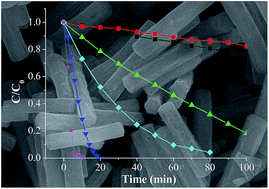
A heterobimetallic complex, zinc titanium glycolate acetate hydrate (Zn2Ti3–GAH), tentatively formulated as Zn2Ti3(OCH2CH2O)4(OCH2CH2OH)5(CH3COO)3·2HOCH2CH2OH·H2O, was synthesized by a room-temperature homogeneous precipitation in ethylene glycol solution. Its chemical composition, crystal structure, morphology, growth mechanism and thermal behaviors were characterized in detail. The precipitated Zn2Ti3–GAH was of a highly crystalline monoclinic phase and porous microrod morphology. As the single source precursor (SSP), Zn2Ti3–GAH was transformed into different phases of zinc titanate via thermal decomposition. With the remaining shape of the microrods, cubic phases of Zn2Ti3O8 and rutile TiO2 (r-TiO2) supported hexagonal phases of ZnTiO3 (h-ZnTiO3) were obtained by calcination at 500 and 700 °C, respectively; while r-TiO2 supported Zn2TiO4 were yielded in the form of dispersed particles or chains at higher temperature (950 °C). Benefiting from the SSP route and the confinement in the specific microrod domains of precursors, the heterostructures of r-TiO2–ZnTiO3 and r-TiO2–Zn2TiO4 were formed during programmable calcination. The studies on photocatalysis by degrading methylene blue (MB) under ultraviolet (UV) irradiation indicated that the as-transformed zinc titanate exhibited enhanced activity. In particular, r-TiO2 supported h-ZnTiO3 displayed the photodegradation reaction rate constant of 0.00163 s−1, which was comparable to that of commercially available Degussa P25 TiO2. This probably related to the more effective charge separation in the r-TiO2–ZnTiO3 heterostructure packed in the microrods.

 Please wait while we load your content...
Please wait while we load your content...