Water-soluble Zn–Ag–In–Se quantum dots with bright and widely tunable emission for biomedical optical imaging†
Abstract
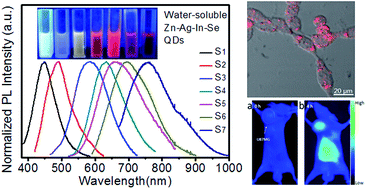
Quantum dots (QDs), as a new fluorescent reagent, should have potential applications in biomedical optical imaging; however their biological applications are limited by the toxicity of the component elements. Hence, in this work, water-soluble Zn–Ag–In–Se (ZAISe) QDs were synthesized without using highly toxic heavy metal elements. The as-prepared quaternary QDs exhibit bright and widely composition-tunable photoluminescence (PL) emission (namely, maximum PL quantum yield (QY) reaching 30%; PL peak from 450 to 760 nm). MTT assay proved that these QDs have much less cytotoxicity than Cd-based QDs. After being further modified by DHLA–PEG–Suc–RGD ligands, water-soluble ZAISe QDs were explored as a fluorescent probe for tumor cell-targeted optical imaging. In vitro and in vivo results demonstrate that ZAISe QDs prepared here should be a promising substitute for Cd-based QDs in biomedical optical imaging.

 Please wait while we load your content...
Please wait while we load your content...