Effect of carboxylated poly(ethylene oxide-b-propylene oxide-b-ethylene oxide) block copolymer on nanostructured unsaturated polyester resin†
D. H. Builes*ab and
A. Tercjak*a
aGroup ‘Materials + Technologies’ (GMT), Department of Chemical and Environmental Engineering, Polytechnic School, University of the Basque Country (UPV/EHU), Pza. Europa 1, 20018 Donostia-San Sebastián, Spain. E-mail: agnieszka.tercjaks@ehu.eus; daniel.builes@andercol.com.co
bPolymeric and Renewable Materials Technological Development Center – DENOVO, Andercol S.A., Medellín, Colombia
First published on 3rd November 2015
Abstract
A carboxylated poly(ethylene oxide-b-propylene oxide-b-ethylene oxide) (PEO-b-PPO-b-PEO) block copolymer was used to fabricate nanostructured unsaturated polyester materials. The commercial PEO-b-PPO-b-PEO block copolymer was carboxylated using succinic and maleic anhydrides in order to improve the miscibility between the carboxylated PEO blocks and the unsaturated polyester matrix and to hinder microphase separation of the PEO blocks during the Reaction Induced Phase Separation (RIPS). Atomic force microscopy (AFM) measurements were employed to detect the well-defined and stable structure of the nanostructured unsaturated polyester matrix. Furthermore, the strong effect of the chemical modification of the PEO-b-PPO-b-PEO block copolymer on the morphology, transparency and mechanical properties of the designed novel nanostructured thermosetting systems based on the unsaturated polyester was also investigated. The designed materials open up a new strategy of preparation of well-defined nanostructured systems modified with chemically modified block copolymers with enhanced toughness.
Introduction
Due mainly to the low cost and properties such as easy handling, resistance to chemicals, corrosion and heat, and rapid strength gain, the technologies offered by unsaturated polyester (UP) resins are widely applied in coatings,1 the automotive industry,2 adhesives,3 and also in the construction industry4 in anchoring grouts, resin mortars and concretes, coatings and lighting sealants. The main drawback of UP thermosetting materials is their high brittleness which is a challenge for many researchers who try to improve the UP thermoset toughness. On the other hand, taking into account that material structure is a fundamental factor of toughness,5–10 a great part of these efforts have been oriented towards the modification of the UP thermoset to reach nanostructured materials with different morphologies and towards the study of its effects on toughening thermosets.In our previous works11–13 it was proved that poly(ethylene oxide-b-propylene oxide-b-ethylene oxide) triblock copolymers (EPE) can be employed as a nanostructuration agent for developing toughened thermosetting materials based on UP. This capability of the EPE is ascribed, on the one hand, to the fact that the initially miscible block copolymer (BCP) microphase separates during the curing process following the Reaction Induced Phase Separation (RIPS) and, on the other hand, to the fact that BCP can form self-assembled micelles before curing which remain in the final cured system.
One of the main difficulties during the nanostructuration process of UP resins is based on the phase heterogeneity in the neat UP generated during curing, which is due mainly to the extreme changes in miscibility at a very low conversion leading to the microphase separation of the St-crosslinked UP oligomers before gelation of the UP resin. This behavior is also one of the main differences between epoxy and UP resins. Moreover, during curing a homogeneous mixture of UP resin and a PEO-based BCP, the decrease of conformational entropy of mixing generates a RIPS of the previous miscible PEO blocks, which in some cases triggers a macrophase separation.13–15 However, contrary to the curing process of epoxy resins, UP resin presents high compositional changes in short time intervals due to the high reaction rate. Thus, due to the RIPS of PEO blocks which proceed until gelation and vitrification, the mesophases obtained are physically “frozen” leading to a thermoset with a fixed morphology in a faster process. Nevertheless, the RIPS of the PEO corona of self-assembled micelles make the micelles unstable, suffering modifications in shape, or in some cases, the micelles are even destroyed. This is one of the reasons for the irregular shape of morphologies obtained when EPE block copolymers are used as a nanostructuration agent for UP matrices.13
Consequently, taking into account that size and shape of the microphase separated domains have a strong relationship with the toughness of cured thermosetting mixtures,16–20 the present work attempts to investigate the changes of an UP matrix structure mixed with the chemically modified lateral blocks of an EPE block copolymer. This chemical modification was carried out using maleic anhydride in order to increase the miscibility between BCP and UP resin and provide unsaturation to the BCP. Likewise, to discern between the chemical and physical effects on the UP matrix modified with the chemically modified EPE, a BCP modified with succinic anhydride was also considered.
The chemical modifications of EPE block copolymers with both anhydrides were studied by means of 1H and 13C nuclear magnetic resonance (1H NMR and 13C NMR, respectively) and Fourier transform infrared spectroscopy (FTIR). The morphology of the designed thermosetting systems was estimated using atomic force microscopy (AFM). In addition, the relationship between morphology and the final optical and mechanical properties of the designed thermosetting systems were also investigated. Additionally, here it should be pointed out that based on our knowledge, this is the first time that the chemically modified BCP was used to nanostructured UP resin.
Experimental
Materials
Linear poly(ethylene oxide-b-propylene oxide-b-ethylene oxide) triblock copolymers with structures E20P69E20 were purchased from Sigma-Aldrich. The BCP has a number average molecular weights (Mn) of 5750 denoted here as EPE. Succinic anhydride 99% (SA) and maleic anhydride 98% (MA) were supplied by Alfa Aesar and used as received. The UP thermosetting precursor was a commercial orthophthalic resin manufactured by Andercol S.A. It contained a prepolymer with a number average molecular weight (Mn) of 1800 g mol−1 and polydispersity index of 3 as determined by gel permeation chromatography, and it was composed of phthalic and maleic anhydrides and dissolved in 36 wt% styrene as cross-linking monomer. Methyl ethyl ketone peroxide (MEKP), supplied by Hegardt S.L., with the trade name Peroxan ME50L was used as polymerization initiator.Carboxylation of EPE
Anhydrides react with the hydroxyl ends of glycols and produce an acid-end group with an ester bridge without water formation.21–23 In the case of succinic or maleic anhydrides and the EPE glycol (see Scheme 1 and S1†), this reaction is fast and exothermic (ΔHrxn = −40 kJ mol−1).24,25Carboxylation of EPE was carried out employing both succinic (SA) and maleic anhydride (MA) to fabricate EPES or EPEM, respectively. The synthesis was performed in a stirred four-necked flask reactor equipped with a heating mantle and N2 inlet. EPE (200 g) was placed into the reactor and temperature was raised until melt. Powdered maleic anhydride was added slowly in stoichiometric relation (6.8 g). Reaction temperature was fixed to 160 °C. The reaction conversion progress was monitored checking acid numbers by acid–base titration employing the standard ASTM D1639.26 Samples of ca. 0.5 g were extracted from the reactor each 15 min, dissolved in deionized water and titrated with 0.105 N KOH solution at room temperature using phenolphthalein as visual pH indicator. After 2 hours of process the reaction efficiencies were equal to 90.3 ± 1.5% and 89.8 ± 2.2% for EPES and EPEM, respectively.
Blending protocol
UP resin was pre-accelerated with 0.3 g OCo per 100 g of resin. Mixtures were prepared adding to the UP resin an adequate amount of EPES or EPEM and stirring it at room temperature (25 °C). Mixtures were denoted taking into account the modifier name and content, e.g., the mixture contained 5 wt% of EPEM and 95 wt% of UP resin was named 5%EPEM. Resin and mixtures without MEKP were named here as nonreactive. The reactive mixtures were prepared adding 1.5 g of MEKP per 100 g of UP. Redox reaction is allowed by the mixture of MEKP and OCo which acts as accelerator of the curing reaction of UP resin.23 OCo produces metal ions which act over MEKP generating peroxy free radicals which act over the unsaturations. Thermosetting sheets were prepared filling molds, made with two flat glasses separated by a U-shaped polytetrafluoroethylene (PTFE), with the reactive mixtures. These mixtures were pre-cured at 25 °C during 24 h in the molds, following a post-curing steps 12 h at 35 °C, 3 h at 85 °C and finally 1 h at 170 °C in a forced convection oven.Characterization techniques
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) was obtained by scans performed at a heating rate of 2 °C min−1 and a frequency of 10 Hz. Rectangular samples of 12.7 mm × 1.0 mm × 30 mm were used.
δ) was obtained by scans performed at a heating rate of 2 °C min−1 and a frequency of 10 Hz. Rectangular samples of 12.7 mm × 1.0 mm × 30 mm were used.Results and discussion
Chemical modifications of EPE
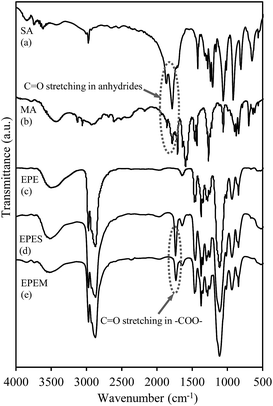
To confirm carboxylation of the EPE block copolymer using SA and MA, the chemical structures of EPES and EPEM were characterized by means of FTIR, 1H NMR and 13C NMR spectroscopies. Fig. 1 shows the FTIR spectra of MA, SA, EPE, EPES and EPEM.As clearly observed in Fig. 1a and b, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of anhydrides exhibited strong vibration absorption peaks at wavenumbers of 1860 and 1780 cm−1. After chemical modification, a comparison between the EPE and the modified BCPs spectra, allowed to conclude that the main difference was linked to the strong vibration absorption peaks at 1736 and 1732 cm−1, related to the formation of C
O group of anhydrides exhibited strong vibration absorption peaks at wavenumbers of 1860 and 1780 cm−1. After chemical modification, a comparison between the EPE and the modified BCPs spectra, allowed to conclude that the main difference was linked to the strong vibration absorption peaks at 1736 and 1732 cm−1, related to the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations in the carboxyl groups of EPES and EPEM, respectively (Fig. 1c–e), confirming a successful modification of EPE.
O stretching vibrations in the carboxyl groups of EPES and EPEM, respectively (Fig. 1c–e), confirming a successful modification of EPE.
These results are in good agreement with results reported for the modification of two different BCPs of the same family26,27 or a polyethylene oxide28 using SA or MA. Moreover, it should be pointed out that the lack of typical peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O related to SA or MA in the investigated systems, indicate high chemical conversion.29
O related to SA or MA in the investigated systems, indicate high chemical conversion.29
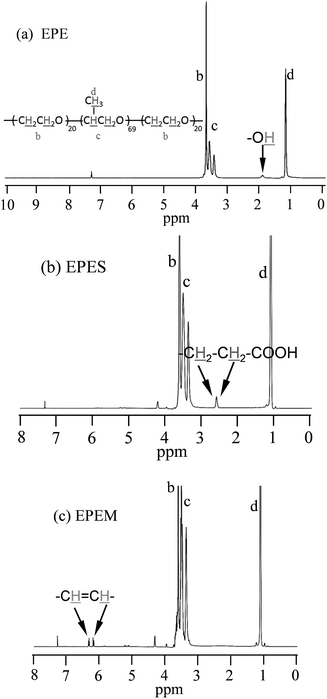
The 1H NMR spectra of EPE, EPES and EPEM are shown in Fig. 2 (see the main chemical shifts in S2†). As can be seen, the characteristic chemical shift corresponding to the hydroxyl group (Fig. 2a) was not observed in both the EPES and EPEM 1H NMR spectra (Fig. 2b and c).
The 1H NMR of the EPES showed a prominent peak at 2.55 ppm corresponding to the methylene protons of the succinic group added to the terminals of EPE, which were not presented in the 1H NMR spectra of EPEM (Fig. 2c), which exhibited signals at 6.2 and 6.4 corresponding to the protons of maleate terminals (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–).29 The 1H NMR spectrum confirmed that chemical modification of the EPE was achieved and that the desired EPES and EPEM were successfully prepared. These results are in good agreement with results reported for the modification of two different BCPs of the same family using SA26 or MA.27 13C NMR measurements were also carried out (see S3†) confirming chemical modification of the EPE block copolymer.
CH–).29 The 1H NMR spectrum confirmed that chemical modification of the EPE was achieved and that the desired EPES and EPEM were successfully prepared. These results are in good agreement with results reported for the modification of two different BCPs of the same family using SA26 or MA.27 13C NMR measurements were also carried out (see S3†) confirming chemical modification of the EPE block copolymer.
Nonreactive mixtures of carboxylated-EPE with UP
Nonreactive UP/EPES and UP/EPEM mixtures with a ratio between components from 100/0 to 50/50 were analyzed. Fig. 3 shows the viscosity changes of these mixtures compared with the UP/EPE mixtures as a function of the BCPs content. As expected, the increase of the BCPs content generated an increase in the viscosity of the mixtures. On the other hand, the measurements showed higher viscosity increases in the carboxylated-EPE mixtures. These viscosity differences could be justified considering mainly the changes in the shape of the terminal groups added, viz. hydroxyl-terminals of EPE were replaced by maleate- or succinate-terminals, whose hook-like shape22,30,31 could increase the entanglements and consequently the chain mobility. A similar effect of the polymer structure on the viscosity has been reported elsewhere.32As can be clearly observed in the digital image in the inset of Fig. 3, mixtures prepared with 50 wt% of chemically modified EPE block copolymers were visually homogeneous and transparent at room temperature. This appearance indicates homogeneity in the visible light scale. Similar behavior was observed for all the investigated liquid mixtures. On the contrary to UP/EPES mixtures, the UP/EPEM mixtures exhibited a slightly pale yellow color, which is a typical characteristic color for maleate. This color was also observed during carboxylation of EPE with MA.
As published by us,11 EPE has the ability to form self-assembled structures when mixed with UP resin. In order to study the changes in the dynamics of chemically modified EPE and UP resin mixtures, DLS measurements of nonreactive 5%EPES and 5%EPEM mixtures were carried out. To compare, 5%EPE mixture was analyzed under the same conditions. Fig. 4 shows the normalized intensity autocorrelation functions, a digital image of the visual appearance, and the intensity weighted decay time distributions plotted in respect to q2τ of some analyzed samples (where q is the scattering angle and τ the decay time).
Fig. 4a shows that analyzed mixtures exhibited similar g2(t) indicating the formation of self-assembled micelles of EPES and EPEM in UP resin at length-scales below the wavelength of visual light. As it can be seen in Fig. 4b, decay time distributions shifted from ca. τq2 = 4.3 × 1012 for 5%EPE to 1.37 × 1013 and to 1.43 × 1013 s m−2 for 5%EPES and 5%EPEM mixtures, respectively. This tendency of decay time distributions to shift to higher relaxation times is indicative of a new regimen with slower dynamics, which can be achieved by particles with an increased impediment to move due to their bigger size or to an increase in the local viscosity,33–35 which agree with the viscosity measurements showed in Fig. 3 which indicated a restriction in the micelles movement.
Miscibility of nonreactive mixtures was studied by means of DSC. Fig. 5 shows the heating scans of the nonreactive UP/EPES and UP/EPEM mixtures.
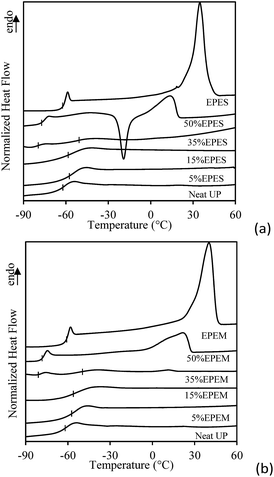 | ||
| Fig. 5 DSC thermograms during heating of nonreactive (a) UP/EPES and (b) UP/EPEM mixtures. Thermograms of EPES and EPEM were included for comparison. | ||
Although not shown here, on the contrary to the nonreactive UP/EPE mixtures investigated previously by us,12 no crystallization was detected for the nonreactive mixtures during cooling. Indeed, the EPES and EPEM exhibited a lower crystallization temperature (20 °C) than the EPE (24 °C).13 The last could be due to crystal imperfections36 or depression in the growth kinetics of crystals attributed to both the higher viscosity of the mixtures (see Fig. 3) and difficulties in the intermolecular arrangement due to steric hindrance31 generated by the shape of the terminal groups of carboxylated-EPE. Additionally, on the contrary to the nonreactive 50%EPEM mixture, the DSC endothermogram of the nonreactive 50%EPES mixture showed an exothermic peak of delayed crystallization confirming once more a higher compatibility in these systems.
The Tm and Tgs of the UP/EPES and UP/EPEM mixtures displayed changes if compared to the Tm and Tgs of the individual components confirming partial miscibility between chemically modified EPE and UP resin. As can be clearly observed, the Tm of both the 50%EPES and 50%EPEM mixtures shifted to a lower temperature if compared with the Tm of the EPES or EPEM, respectively, with the Tm corresponding to a 50%EPES mixture lower than the Tm corresponding to the 50%EPEM mixture (14 and 22 °C, respectively). This fact indicated a higher miscibility for UP/EPES systems than for the UP/EPEM systems. Mixtures containing 5 and 15 wt% of BCPs did not show any endothermic transition.
Compared to the UP/EPE system, the observed changes in the miscibility of the UP/carboxylated-EPE can be related to the fact that the carboxyl functional group has a higher association degree through hydrogen bonding since their –OH bonds are more strongly polarized if compared with the hydroxyl functional group37 allowing for higher BCP–UP interactions. Furthermore, it could be also expected that the maleate groups that are part of the UP oligomer chains structure could promote interactions with the maleate or succinate groups of carboxylated-EPE.
Thermal behavior of UP/EPES and UP/EPEM cured systems
The investigated thermosetting systems were homogeneous and transparent indicating the lack of macrophase separation in the systems modified with both EPES and EPEM. Fig. 6 shows the thermal behavior of the UP/EPES and UP/EPEM cured systems. The values of thermal transitions and degree of crystallization (Xc) for the investigated thermosetting systems are summarized in Table 1.The Tc of thermosetting systems modified with 50 wt% of EPES or EPEM shifted to lower temperatures if compared with the Tc of respective chemically modified BCPs. Simultaneously, the enthalpy of the crystallization process decreased for both thermosetting systems being much notable for EPEM than for EPES (Fig. 6a).
As can be seen, the curing process changed the thermal characteristics of crystallization, e.g. the Xc of 50%EPEM changed from 53 to 25 and the Tm from 22 to 34 °C after network formation. A similar effect was also reported in the literature by Guo et al.38 This depression in the growth kinetics and the increase of Tm can be attributed to the reduction of chain mobility due to the fact that a crosslinked UP network exhibits a higher Tg than the thermosetting precursor,38 viz. in liquid mixtures, the chains have the freedom to find each other, form hydrogen bonding and generate crystals. Likewise, the crystals can be easily destroyed in nonreactive mixtures due to the higher chain mobility and consequently lower temperature that is required to melt the crystals. It is worth mentioning that the difference between the Tc of 50%EPES and the 50%EPEM cured systems (4 and −31 °C, respectively) could be due to the lower chain mobility in the 50%EPEM cured system as a consequence of the crosslinked reaction between the unsaturation of EPEM and UP resin.
Under measurement conditions, the UP cured systems with 5 and 15 wt% BCPs did not exhibit any crystallization transition neither during cooling nor heating scans, which implies that chemically modified PEO blocks could remain amorphous in cured systems as was also observed for the 5%EPE and 15%EPE cured systems.11,13 The Tg-BCPs of the investigated cured systems shifted after curing to values almost equal to the Tgs of neat BCPs. This effect is related to the phase separation in the designed thermosetting systems and the homopolymerization of styrene inside micelles.11
The dynamic mechanical behavior of thermosetting systems was also analyzed. The temperature dependence of loss factor, tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, and storage modulus, E′, for UP and 5 and 15 wt% UP/EPES and the UP/EPEM cured systems are shown in Fig. 7. It should be mentioned that a dynamic mechanical analysis was not done for the 50 wt% BPC cured systems since in this case different sample dimensions and/or different measurement conditions were required to obtain representative results. For this reason these results were not taken into account.
δ, and storage modulus, E′, for UP and 5 and 15 wt% UP/EPES and the UP/EPEM cured systems are shown in Fig. 7. It should be mentioned that a dynamic mechanical analysis was not done for the 50 wt% BPC cured systems since in this case different sample dimensions and/or different measurement conditions were required to obtain representative results. For this reason these results were not taken into account.
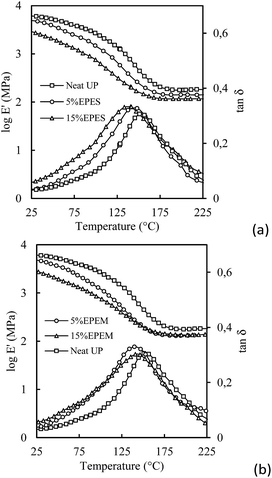 | ||
Fig. 7 Variation of storage modulus, E′, and loss factor, tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, upon EPES (a) and EPEM (b) amount for neat UP (–□–), and 5 wt% (–○–) and 15 wt% (–△–) modified thermosets. δ, upon EPES (a) and EPEM (b) amount for neat UP (–□–), and 5 wt% (–○–) and 15 wt% (–△–) modified thermosets. | ||
As expected, the temperature variations of glass modulus, Eg, and rubber modulus, Er, of the cured systems decreased with the increase of temperature and the BCPs content if compared with the neat UP matrix. The same behavior was detected for the thermosetting systems modified with EPE.13 However, the 15%EPEM mixture displayed no decrease in plateau of Er if compared with the 5%EPEM, which was higher if compared with the Er of 15%EPES indicating a higher thermal stability of the storage modulus. This phenomenon could be due to a crosslinked reaction between the unsaturation of EPEM and UP resin.
The damping properties of UP cured mixtures were analyzed by means of tan δ peaks and were used to analyze the interface between the microphase separated domains and the UP-rich matrix. Fig. 7a shows an increase in the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ curves after being mixed with EPES indicating that some part of the UP-rich network had a higher mobility39,40 than the neat UP matrix. This behavior indicates a mixing between the modified-PEO lateral blocks and the micro-separation PPO-rich phase at the boundary between the microseparation PPO-rich phase and the crosslinked UP matrix. Chemically modified PEO blocks have a low Tg and in the amorphous phase they can plasticize the glassy UP-rich matrix. Similar behavior was also found for the UP/EPE cured systems.11–13 As clearly seen in Fig. 7a, the height of tan
δ curves after being mixed with EPES indicating that some part of the UP-rich network had a higher mobility39,40 than the neat UP matrix. This behavior indicates a mixing between the modified-PEO lateral blocks and the micro-separation PPO-rich phase at the boundary between the microseparation PPO-rich phase and the crosslinked UP matrix. Chemically modified PEO blocks have a low Tg and in the amorphous phase they can plasticize the glassy UP-rich matrix. Similar behavior was also found for the UP/EPE cured systems.11–13 As clearly seen in Fig. 7a, the height of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peaks increased and the temperature in the maximum of the tan
δ peaks increased and the temperature in the maximum of the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peaks decreased with the increase of the EPES content. This phenomenon can be related to the increased chain mobility due to the plasticization effect of the EPES. Furthermore, the wide temperature range over which tan
δ peaks decreased with the increase of the EPES content. This phenomenon can be related to the increased chain mobility due to the plasticization effect of the EPES. Furthermore, the wide temperature range over which tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ is affected presumably reflects a gradient in composition in the vicinity of the miscible PEO-modified blocks of separated domains (i.e. micelles corona). On the contrary, the 15%EPEM system displayed a reduction in the magnitude of tan
δ is affected presumably reflects a gradient in composition in the vicinity of the miscible PEO-modified blocks of separated domains (i.e. micelles corona). On the contrary, the 15%EPEM system displayed a reduction in the magnitude of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ peak and a shift of the maximum to a higher temperature if compared to the 5%EPEM and the 15%EPES cured systems. This hindering energy dissipation process of thermosetting molecules could be associated with a constrained chain mobility generated by a chemical fixing of the EPEM with the UP-rich matrix during the crosslinking process. Taking into account the DMA results, one could conclude that the presence of the covalent bonds between the EPEM and UP-rich matrix can hinder its deformation, if compared with the UP systems modified with EPE or EPES, leading to an increase of the Tg of thermosets modified with the same amount of BCPs and confirming the stronger interface between the UP-rich and EPEM-rich phases after curing. The differences observed between the Tgs of the UP-rich matrix in the 15%EPES and the 15%EPEM cured systems could be similar to the phenomenon observed by Dong et al. for reactive BCPs,41 and explained as a compensation of the plasticization effect with an increase of the crosslinking density provoked by the reactive blocks.
δ peak and a shift of the maximum to a higher temperature if compared to the 5%EPEM and the 15%EPES cured systems. This hindering energy dissipation process of thermosetting molecules could be associated with a constrained chain mobility generated by a chemical fixing of the EPEM with the UP-rich matrix during the crosslinking process. Taking into account the DMA results, one could conclude that the presence of the covalent bonds between the EPEM and UP-rich matrix can hinder its deformation, if compared with the UP systems modified with EPE or EPES, leading to an increase of the Tg of thermosets modified with the same amount of BCPs and confirming the stronger interface between the UP-rich and EPEM-rich phases after curing. The differences observed between the Tgs of the UP-rich matrix in the 15%EPES and the 15%EPEM cured systems could be similar to the phenomenon observed by Dong et al. for reactive BCPs,41 and explained as a compensation of the plasticization effect with an increase of the crosslinking density provoked by the reactive blocks.
Morphology and transparency of UP/EPES and UP/EPEM cured systems
The morphology of the cured systems was investigated by means of AFM. Fig. 8 shows the morphology of the thermosetting system modified by the different contents of the EPES and EPEM. For comparison, the UP/EPE cured system was included. The investigated systems were transparent, as visualized in the inset at the top of each image in Fig. 8 indicating the absence of macrophase separation.As clearly seen in Fig. 8a, the 5%EPES and 5%EPEM cured systems exhibited phase-separated domains more spherical and more segregated, and markedly smaller if compared to those observed for the 5%EPE cured system. It was observed that increasing the EPES content to 15 wt% led to longer structures which tended to be worm-like. Moreover, for this system more agglomerated domains can be detected if compared with the 15%EPE cured system. Regarding the 15%EPEM cured system, a strong change in morphology was observed if compared with the 15%EPE and 15%EPES cured systems. These last two thermosetting mixtures depicted worm-like structures, while the 15%EPEM cured system tended to maintain a sphere-like morphology.
The last morphology changes can be justified considering that the presence of a carboxyl group in the modified EPE can considerably increase the possibilities of conformational structures due to hydrogen bonding with the hydroxyl, carbonyl and carboxyl groups of the UP resin.42,43 This could affect the self-assembly of the carboxylated-EPE leading to the strong changes observed in their final morphology if compared with the morphology of the UP/EPE cured systems. A similar work on the self-assembly of the BCP ended in carboxylic group was reported by Gong44 and Liu.45 Moreover, as can be clearly observed in Fig. 8b, the UP/EPES thermoset displayed a lower segregation of microphase separated domains, despite the higher miscibility effect of the EPES in nonreactive mixtures (see Fig. 5). The last was corroborated by both the digital images of transparency shown as insets of Fig. 8b where a lower transparency of the UP/EPES thermoset was observed, and in Fig. 3 where to the naked eye, nonreactive mixtures exhibited almost equal transparency. Therefore, this difference caused by the curing process could be attributable to a crosslinking process between the unsaturation of EPEM and UP resin. This property of the EPEM permitted the stabilization of the micelles by means of inducing the crosslinking reaction of the corona blocks. The stabilization of the EPE micelles by means of the induced crosslinking reaction has been also reported by other authors.46–48 Fig. 8c shows the morphology of the thermosetting mixtures modified with 50 wt% BCPs. As can be easily detected, also in this case significant differences in the morphology of the systems modified with EPE and carboxylated-EPE were distinguishable. The 50%EPEM cured system tended to form less dense worm-like structures than the 50%EPE and 50%EPES systems. A higher dispersion of phase-separated domains than in the 50%EPES cured system was also detected, as corroborated by the digital image of transparency shown in the top inset of Fig. 8c2 and c3, where the higher transparency of the EPEM modified thermosetting systems was observed. A proposed sequence of nanostructuration in the UP/EPE and UP/EPEM cured systems can be explained by means of the schematic representation shown in Scheme 2.
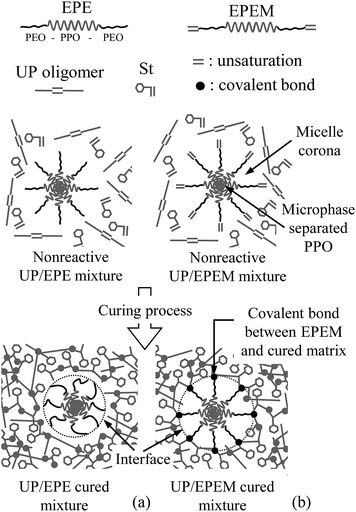 | ||
| Scheme 2 Schematic description of the differences achieved in UP/EPE and UP/EPEM mixtures after curing. | ||
After curing, the PEO miscible blocks of EPE in a UP/EPE mixture (Scheme 2a) underwent a reaction induced phase separation of PEO blocks,12 generating a reduced micelle corona and a weak EPE matrix interface. On the contrary, due to the EPEM unsaturation, the UP/EPEM mixtures could decrease the degree of RIPS due to a stronger interaction with the crosslinked matrix in the interface (Scheme 2b).
Mechanical properties of UP/carboxylated-EPE cured systems
The mechanical properties of the UP/EPES and UP/EPEM cured systems were measured by means of the three-point bending and fracture toughness tests. The values of the flexural modulus, E, flexural stress at break, σ, and flexural strain, ε, are reported in Fig. 9 as a function of the BCPs and their content. Table 2 summarizes the mechanical properties of the investigated thermosetting systems.| System | E (GPa) | σf (MPa) | ε (mm mm−1) | KIc (MPa m0.5) | GIc (J m−2) |
|---|---|---|---|---|---|
| Neat UP | 4.079 ± 0.036 | 166.1 ± 4.0 | 0.039 ± 0.001 | 0.51 ± 0.01 | 54 ± 3 |
| 5%EPE | 3.506 ± 0.015 | 139.2 ± 4.6 | 0.037 ± 0.002 | 0.66 ± 0.03 | 104 ± 10 |
| 5%EPES | 3.469 ± 0.042 | 125.4 ± 4.5 | 0.035 ± 0.002 | 0.65 ± 0.03 | 102 ± 10 |
| 5%EPEM | 3.475 ± 0.060 | 125.6 ± 4.5 | 0.036 ± 0.002 | 0.60 ± 0.01 | 88 ± 4 |
| 15%EPE | 2.155 ± 0.043 | 78.4 ± 1.9 | 0.037 ± 0.002 | 0.72 ± 0.02 | 205 ± 11 |
| 15%EPES | 2.108 ± 0.035 | 66.2 ± 2.7 | 0.036 ± 0.003 | 0.54 ± 0.02 | 115 ± 8 |
| 15%EPEM | 2.074 ± 0.024 | 92.0 ± 8.4 | 0.049 ± 0.002 | 0.78 ± 0.02 | 242 ± 22 |
As it is shown in Fig. 9a, E decreased with the increase of the BCP content. No marked differences were observed if comparing the effects of 5 wt% content of the EPE, EPES or EPEM. Despite the 15%EPEM cured system exhibited the lowest E, it presented the highest value of σ and ε. This result could be related to the damping behavior observed in the DMA analysis (Fig. 7b), and is probably attributed to an improvement of the interface between phase separated domains and the matrix generated by the formation of covalent bonds between carboxylated-PEO blocks and UP-rich matrix.
A similar effect has been reported by Dong et al.,41 where a UP matrix modified with a reactive BCP leads to a high tensile strength despite a Young's modulus reduction. They also demonstrated that mechanical properties depended on the morphology of the investigated systems and interfacial properties between the UP-rich matrix and the BCP-rich phase.
Taking into account the morphology analysis, the 15%EPEM system showed a sphere-like structure with a higher interdomain distance. The relationship between the mechanical properties and the sphere-like or worm-like morphology was also discussed by Dean et al.16
As can be seen in Fig. 9b, the toughness of the UP matrix was clearly improved by the addition of both EPES and EPEM. No significant differences in toughness between the thermosetting systems modified with 5 wt% of BCPs were observed. However, the 5%EPEM cured system exhibited slightly lower toughness than the 5%EPE and 5%EPES. These results are in good agreement with the morphology generated in the final nanostructured thermosetting systems. In the case of the 5 wt% modified systems one could conclude that there was a relationship (R) between the diameter of the phase-separated domains (δ) and the interdomain distance (ρ). In the case of the 5%EPE system, R = δ/ρ was higher as well as KIc, and in the case of the 5%EPEM, R was lower (see the arrows in Fig. 8a) as well as KIc. The last relationship between morphology and fracture toughness was also reported in the literature for the epoxy systems modified with different BCPs.16 On the contrary, when the BCPs content increased to 15 wt%, the toughness of the EPEM modified thermosets presented an improvement of ca. 18 and 110% of GIc if compared with the EPE and EPES modified thermosets. At this BCP content, a close relationship between morphology and toughness was also observed since the 15%EPES and the 15%EPEM showed the lowest and the highest toughness and also the lowest and the highest spreading of microphase separated domains, respectively (see Fig. 9b and 8b).
This improvement in toughness could be explained considering an improved interface interaction between the micelles corona of the EPEM and the surrounding matrix due to the covalent bonds, as was explained above. Similar improvements in fracture toughness for the epoxy matrices modified with reactive BCPs were reported by Dean et al.16 and Rebizant et al.17
Conclusions
According to the FTIR and 1H NMR, the carboxylation of the EPE was successfully achieved by means of succinic or maleic anhydride, and the effects on the nonreactive mixtures and on the cured systems based on UP resin were investigated and compared with the UP/EPE mixtures.Changes in the properties of nonreactive and cured systems were also studied. The DSC and DLS results showed that nonreactive UP/EPES and UP/EPEM mixtures exhibited a weak tendency to reduce the crystallization and the dynamics of the systems if compared with UP/EPE mixtures. Damping behavior revealed that, on the contrary to UP/EPES cured systems, the UP/EPEM cured systems tended to increase the Tg of matrix with an increase of BCP content from 5 to 15 wt%. The differences in the BCP content affected also the final morphology of the designed thermosetting systems. The worm-like domains detected for the 15%EPE and the agglomeration detected for the 15%EPES cured mixture were changed to a well-segregated microphase separated sphere-like domain for the 15%EPEM.
Regarding mechanical properties, the 15%EPEM cured system exhibited the highest fracture toughness (an improvement of more than 50% if compared with neat UP) and flexural strain (an increase of almost 25% if compared with neat UP).
In conclusion, in this work the novel pathway to designed nanostructured UP based materials was verified. The proposed chemical modification of the EPE led to controlling the final morphology of the nanostructured thermosetting systems and allowed a better understanding of the relationship between morphology, interface and the improved mechanical properties of unsaturated polyester based materials.
Acknowledgements
This work was supported by the Spanish Ministry of Economy and Competitiveness and European Union funded project (MAT2012-31675) and by the Basque Government funded Grupos Consolidados project (IT776-13). A. T. acknowledges MICINN for Ramón y Cajal program (RYC-2010-05592). Moreover, we are grateful to the ‘Macrobehavior-Mesostructure-Nanotechnology’ and Nuclear Magnetic Resonance (NMR) SGIker units of the UPV/EHU. D. H. B. gratefully acknowledges Andercol S.A. and Group ‘Materials + Technologies' of the University of the Basque Country for their support. The authors wish to also thank to Hugo Hernández from DENOVO-Andercol for fruitful discussions.References
- E. D. Cohen and E. B. Gutoff, Coating Methods, Survey, in Encyclopedia of Polymer Science and Technology, John Wiley & Sons, 2001 Search PubMed
.
- N. Boyard, C. Serre and M. A. Vayer, J. Appl. Polym. Sci., 2007, 103, 451 CrossRef CAS
.
- P. J. Chenier, in Survey of Industrial Chemistry, Springer, New York, 3rd edn, 2002 Search PubMed
.
- M. H. Irfan, in Chemistry and Technology of Thermosetting Polymers in Construction Applications, Springer, Dordrecht, 1998 Search PubMed
.
- U. G. K. Wegst and M. F. Ashby, Philos. Mag., 2004, 84, 2167 CrossRef CAS
.
- E. Serrano, P. Gerard, F. Lortie, J. P. Pascault and D. Portinha, Macromol. Mater. Eng., 2008, 293, 820 CrossRef CAS
.
- C. Ocando, A. Tercjak, M. D. Martín, J. A. Ramos, M. Campo and I. Mondragon, Macromolecules, 2009, 42, 6215 CrossRef CAS
.
- K. de la Caba, P. Guerrero, J. Gavalda and I. Mondragon, J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 1677 CrossRef CAS
.
- M. Bakar and F. Djaider, J. Thermoplast. Compos. Mater., 2007, 20, 53 CrossRef CAS
.
- M. Larrañaga, E. Serrano, M. D. Martín, A. Tercjak, G. Kortaberria, K. de la Caba, C. Riccardi and I. Mondragon, Polym. Int., 2007, 56, 1392 CrossRef
.
- D. H. Builes, H. Hernández, I. Mondragon and A. Tercjak, J. Phys. Chem. C, 2013, 117, 3563 CAS
.
- D. H. Builes, J. P. Hernández-Ortiz, M. A. Corcuera, I. Mondragon and A. Tercjak, ACS Appl. Mater. Interfaces, 2014, 6, 1073 CAS
.
- D. H. Builes, A. Tercjak and I. Mondragon, Polymer, 2012, 53, 3669 CrossRef CAS
.
- N. Boyard, C. Sinturel, M. Vayer and R. Erre, J. Appl. Polym. Sci., 2006, 102, 149 CrossRef CAS
.
- X. Li, W. Fu, Y. Wang, T. Chen, X. Liu, H. Lin, P. Sun, Q. Jin and D. Ding, Polymer, 2008, 49, 2886 CrossRef CAS
.
- J. M. Dean, R. B. Grubbs, W. Saad, R. F. Cook and F. S. Bates, J. Polym. Sci., Part B: Polym. Phys., 2003, 41, 2444 CrossRef CAS
.
- V. Rebizant, A. S. Venet, F. Tournilhac, E. Girard-Reydet, C. Navarro, J. P. Pascault and L. Leibler, Macromolecules, 2004, 37, 8017 CrossRef CAS
.
- J. M. E. Dean, P. M. Lipic, R. F. Cook and F. S. Bates, J. Polym. Sci., Part B: Polym. Phys., 2001, 39, 2996 CrossRef CAS
.
- J. Liu, H. J. Sue, Z. J. Thompson, F. S. Bates, M. Dettloff, G. Jacob, N. Verghese and H. Pham, Macromolecules, 2008, 41, 7616 CrossRef CAS
.
- J. Liu, Z. J. Thompson, H. J. Sue, F. S. Bates, M. A. Hillmyer, M. Dettloff, G. Jacob, N. Verghese and H. Pham, Macromolecules, 2010, 43, 7238 CrossRef CAS
.
- R. Jedlovcnik, A. Sebenik, J. Golob and J. Korbar, Polym. Eng. Sci., 1995, 35, 1413 CAS
.
- Y. S. Yang and J. P. Pascault, J. Appl. Polym. Sci., 1997, 64, 133 CrossRef CAS
.
- J. P. Pascault, H. Sautereau, J. Verdu and R. J. J. Williams, in Thermosetting polymers, Marcel Dekker, New York, 2002 Search PubMed
.
- M. Shah, E. Zondervan and A. B. de Haan, J. Appl. Sci., 2010, 10, 2552 Search PubMed
.
- M. Shah, E. Zondervan, M. L. Oudshoorn and A. B. de Haan, Chem. Eng. Process., 2011, 50, 747 CrossRef CAS
.
- W. Zhang, K. Gilstrap, L. Wu, B. K. C. Remant, M. A. Moss, Q. Wang, X. Lu and X. He, ACS Nano, 2010, 4, 6747 CrossRef CAS PubMed
.
- N. N. Rozik, S. L. Abd-El Messieh and K. N. Abd-El Nour, J. Appl. Polym. Sci., 2010, 115, 1732 CrossRef CAS
.
- İ. Özen, K. Dirnberger, C. Rustal, H. G. Fritz and C. D. Eisenbach, Polym.-Plast. Technol. Eng., 2013, 52, 101 CrossRef
.
- W. Zhang, Z. Du, C. H. Chang and G. Wang, J. Colloid Interface Sci., 2009, 337, 56 Search PubMed
.
- T. Felthouse, J. Burnett, B. Horrell, M. Mummey and Y.-J. Kuo, Maleic Anhydride, Maleic acid and fumaric acid, in Van Nostrand's Encyclopedia of Chemistry, John Wiley & Sons, New York, 2001 Search PubMed
.
- G. Odian, in Principles of polymerization, John Wiley & Sons, Canada, 4th edn, 2004 Search PubMed
.
- A. Jabbarzadeh, J. D. Atkinson and R. I. Tanner, Macromolecules, 2003, 36, 5020 CrossRef CAS
.
- A. F. Kostko, J. L. Harden and M. A. McHugh, Macromolecules, 2009, 42, 5328 CrossRef CAS
.
- W. Schärtl, in Light Scattering from Polymer Solutions and Nanoparticle Dispersions, Springer, Berlin, 2007 Search PubMed
.
- M. Shibayama, T. Karino and S. Okabe, Polymer, 2006, 47, 6446 CrossRef CAS
.
- B. Zhang, Y. Li, P. Ai, Z. Sa, Y. Zhao, M. Li, D. Wang and K. Sha, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5509 CrossRef CAS
.
- J. D. Roberts and M. C. Caserio, in Basic Principles of Organic Chemistry, W. A. Benjamin Inc., Menlo Park, 2nd edn, 1977 Search PubMed
.
- Q. Guo and G. Groeninckx, Polymer, 2001, 42, 8647 CrossRef CAS
.
- J. Ma, M. S. Mo, X. S. Du, P. Rosso, K. Friedrich and H. C. Kuan, Polymer, 2008, 49, 3510 CrossRef CAS
.
- J. Liu, H. J. Sue, Z. J. Thompson, F. S. Bates, M. Dettloff, G. Jacob, N. Verghese and H. Pham, Acta Mater., 2009, 57, 2691 CrossRef CAS
.
- J. P. Dong, J. H. Lee, D. H. Lai and Y. J. Huang, J. Appl. Polym. Sci., 2006, 100, 867 CrossRef CAS
.
- T. R. Shattock, K. K. Arora, P. Vishweshwar and M. J. Zaworotko, Cryst. Growth Des., 2008, 8, 4533 CAS
.
- T. Beyer and S. L. Price, J. Phys. Chem. B, 2000, 104, 2647 CrossRef CAS
.
- X. Gong, Phys. Chem. Chem. Phys., 2013, 15, 10459 RSC
.
- X. Liu, Xi. Gong, Q. Hu and Y. Li, RSC Adv., 2015, 5, 64192 RSC
.
- T. F. Yang, C. N. Chen, M. C. Chen, C. H. Lai, H. F. Liang and H. W. Sung, Biomaterials, 2007, 28, 725 CrossRef CAS PubMed
.
- N. Li, J. Wang, X. Yang and L. Li, Colloids Surf., B, 2011, 83, 237 CrossRef CAS PubMed
.
- S. H. Lee, Y. Lee, S. W. Lee, H. Y. Ji, J. H. Lee, D. S. Lee and T. G. Park, Acta Biomater., 2011, 7, 1468 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18290e |
| This journal is © The Royal Society of Chemistry 2015 |