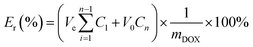Synthesis, characterization and self-assembly behavior of zwitterionic amphiphilic triblock copolymers bearing pendant amino acid residues
Xiaohong Shang,
Xiaoshan Fan,
Shaohui Yang,
Zhengzheng Xie,
Yuming Guo and
Zhiguo Hu*
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, Henan Engineering Laboratory of Chemical Pharmaceuticals & Biomedical Materials, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, P. R. China. E-mail: zghu@htu.cn
First published on 3rd November 2015
Abstract
Zwitterionic amphiphilic triblock copolymers bearing pendant amino acid residues poly(allyl glycidyl ether)/cysteine-b-poly(ε-caprolactone)-b-polyethylene glycol (PAGE/cys-b-PCL-b-PEG) were successfully synthesized via a combination of ring-opening polymerization (ROP), condensation reaction and click reaction. The composition and structure of these copolymers were characterized by gel permeation chromatography (GPC) and 1H nuclear magnetic resonance (1HNMR) spectroscopy. The self-assembly behavior of the copolymers was investigated by fluorescence (FL), low temperature transmission electron microscopy (cryo-TEM), dynamic light scattering (DLS) and zeta potential. The CMC was 0.20 mg mL−1 (PAGE3k/cys-b-PCL6k-b-PEG3.5k) and 0.47 mg mL−1 (PAGE1k/cys-b-PCL3k-b-PEG2k). The copolymers could self-assemble into spherical micelles with diameters of 26 nm (PAGE3k/cys-b-PCL6k-b-PEG3.5k) and 19 nm (PAGE1k/cys-b-PCL3k-b-PEG2k). Doxorubicin (DOX), an anticancer drug, was encapsulated into the micelles to evaluate the drug release ability. The biocompatibility of these copolymers was evaluated with relatively lower cytotoxicity. Therefore, these zwitterionic amphiphilic triblock copolymers are expected to emerge as promising nanocarriers for various drug deliveries.
Introduction
Nanoparticles formed by amphiphilic block copolymers in selective solution have emerged as promising nanocarriers for various drug deliveries1–3 due to their size-dependent chemical and physical properties4 and excellent characteristics of amphiphilic block copolymers.5 However, traditional nanoparticles would be cleared rapidly from the blood stream by the reticuloendothelial system (RES) or mononuclear phagocytic system (MPS).6 It is believed that nonspecific protein adsorption is the first interaction event that occurs at the interface between polymers and human blood.7 Therefore, it is necessary to develop polymeric nanoparticles with antifouling surfaces.Currently, the most prevalent antifouling materials are ethylene glycol-based materials such as poly (ethylene glycol) (PEG) and oligo (ethylene glycol) (OEG).8–10 However, the degradation of ethylene glycol-based materials by oxidation can result in loss of antifouling properties upon prolonged usage or storage.11 Other antifouling materials reported in recent years include hydrophilic polymers such as poly(acrylamide),12 polysaccharides,13 polypeptoids,14,15 and zwitterionic-based materials like poly(carboxybetaine acrylamide) (pCBAA),16 poly(carboxybetaine methacrylate) (pCBMA),17 poly(sulfobetaine methacrylate) (pSBMA),18,19 and poly(2-methacryloyloxyethyl phosphorylcholine).20 Whereas, zwitterionic materials have drawn more attention due to their antifouling properties, which are attributed to their strong hydration capacity dictated by electrostatic interactions between zwitterions and water.21
Amino acids are widely distributed and play an important role in living organisms. Moreover, they are also natural zwitterions. A few previous works have explored the zwitterionic structure-responsible properties of the amino acid-containing materials or amino acid-based zwitterionic materials in various applications. Shiraishi reported that the microspheres of copolymer of zwitterionic O-methacryloyl-L-serine (SerMA) and methyl methacrylate (MMA) had reduced adsorption of albumin and fibrinogen, as compared to the PMMA microspheres.22 An antifouling zwitterionic surface was realized by functionalizing silica nanoparticles with cysteine via its thiol group, which displayed enhanced stability (i.e., less particle aggregation) in solutions of lysozyme, bovine serum albumin (BSA) and diluted human serum.23 In another study, short-chain lysine was grafted onto the surface of the polyacrylonitirile porous filtration membrane, which lead to less BSA and lysozyme adsorption.24 Besides, Liu et al. reported that pSerMA-grafted Au surfaces can strongly suppress not only single protein adsorption, but also adsorptions from full human blood serum and plasma.25 Therefore, amino acids have become a relatively competitive candidate as antifouling materials in the blood stream.
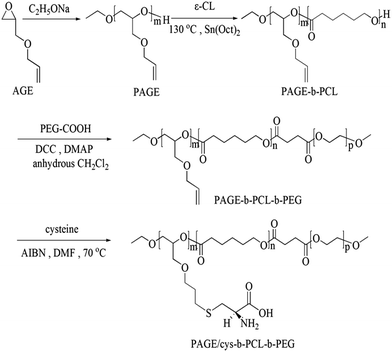
In present study, zwitterionic amphiphilic triblock copolymers PAGE/cys-b-PCL-b-PEG were synthesized as shown in Scheme 1.
Among which, zwitterionic polymer segments PAGE bearing cysteine residues and PEG could form hydrophilic surface to ensure nanoparticles stabilization26,27 in aqueous media and inhibit aggregation by interactions with proteins in the blood flow. Moreover, the self-assembly behavior, drug release profile and the in vitro cytotoxicity assay of the copolymers were also studied.
Experimental
Materials and methods
Allyl glycidyl ether (AGE) was dried over CaH2 for 24 h at room temperature and distilled under reduced pressure. Tin(II) bis (2-ethylhexanoate) (Sn(Oct)2) was purchased from Alfa Aesar and used as received. ε-caprolactone was obtained from Glaco Ltd and used without further purification. L-Cysteine was obtained from Beijing Biodee Biotechnology Ltd. mPEG-OH (Mn = 2000 and 3500) was purchased from Sigma and dried at 80 °C under vacuum for 24 h before use. Dimethylformamide (DMF) was dried by refluxing over CaH2 and was distilled under reduced pressure before use. Dialysis tubing (molecular weight cutoff 2000, 3500, 10k Da) was purchased from Shanghai Green Bird & Technology. mPEG-COOH (Mn = 2000 and 3500) was synthesized from mPEG-OH according to literature procedures.281HNMR spectra were measured on a Bruker-400 NMR instrument in CDCl3, DMSO-d6 using TMS as internal standard. Molecular weight and molecular weight distribution of the polymer samples were determined by gel permeation chromatography (GPC) using a Waters 1515 apparatus equipped with three columns (Waters Styragel HR3, HR4, HR5), a Waters 1515 ISOCRATIC HPLC PUMP, a REFRACTOMETER 2414 detector in series (eluent, THF; flow rate, 1.0 mL min−1). The GPC chromatogram was calibrated against standard polystyrene samples at 35 °C. Fluorescence spectra were recorded on a CARY Eclipse FL spectrometer in a right-angle geometry (90 collecting optics) at 25 °C. The cryo-TEM was performed to characterize the morphology of the micelles. A small droplet of solution was placed on a holey carbon film, which was further supported on a TEM copper grid. The droplet was then blotted to form a film of solution on the grid and allowed to equilibrate. The sample-loaded grid was then plunged into liquid nitrogen, and the solvent was vitrified. The sample was then transported under liquid nitrogen and mounted onto the cryo holder of the TEM. During the entire measurement, the sample was under vacuum. All the images were acquired in a Tecnai Spirit (120 kV TEM), operating at 120 kV and equipped with a Gatan CT3500 cryo holder. The temperature of the sample was maintained at −178 °C. A FEI Eagle CCD camera was used to record the images, which were then processed using FEI Xplore3D software. The diameter distribution and zeta potential of the micelles were measured by using Malvern Zetasizer nano-ZS-90.
Synthesis of poly(allyl glycidyl ether) (PAGE) homopolymers29
Under nitrogen, 0.50 g (7.35 mmol) of anhydrous C2H5ONa and 2 mL of anhydrous xylene were introduced into a flame-dried and nitrogen-purged Schlenk tube equipped with a stirrer, followed by sonication for 15 min. When C2H5ONa was dispersed evenly into xylene, 22.43 g (0.19 mol) of AGE was injected using a glass syringe. The reaction mixture was heated at 100 °C for 24 h. After that, 0.42 mL (7.38 mmol) of acetic acid was added to stop the polymerization reaction, and then the system was cooled to room temperature. The crude PAGE was dissolved in CH2Cl2, dried with anhydrous Na2CO3 overnight and then filtered. The filtrate was concentrated under reduced pressure, and the solution was precipitated with hexane to give a light yellow viscous liquid. The results were summarized in Table 1.| Sample | Initiator (g) | Monomer (g) | Molar ratio (I/M) | Mna | Mnb | Mnc | Mw/Mnc |
|---|---|---|---|---|---|---|---|
| a Calculated from the molar ratio of monomer and initiator.b Determined by 1HNMR in CDCl3.c Measured by GPC in THF with polystyrene as calibration standard. | |||||||
| PAGE1k | 0.50 | 7.89 | 1/9.5 | 1000 | 912 | 1997 | 1.10 |
| PAGE3k | 0.50 | 22.43 | 1/27 | 3000 | 2736 | 5070 | 1.06 |
| PAGE1k-b-PCL3k | 1.00 | 3.38 | 1/27 | 4000 | 3762 | 6424 | 1.48 |
| PAGE3k-b-PCL6k | 1.00 | 2.50 | 1/60 | 9000 | 9006 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730 730 |
1.44 |
| PAGE1k-b-PCL3k-b-PEG2k | 5762 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040 |
1.36 | ||||
| PAGE3k-b-PCL6k-b-PEG3.5k | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 506 506 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170 170 |
1.30 | ||||
PAGE3k: 1HNMR, δ (CDCl3, ppm): 1.21 (t, 3H, CH3CH2O–), 3.47–3.67 (m, 120H, –CH2CH(CH2O–)O–), 4.01 (d, 48H, –OCH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.20 (d, 24H, –CH
CH2), 5.20 (d, 24H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.28 (d, 24H, –CH
CH2), 5.28 (d, 24H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.91 (m, 24H, –CH
CH2), 5.91 (m, 24H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). GPC: Mn = 5070, Mw/Mn = 1.06.
CH2). GPC: Mn = 5070, Mw/Mn = 1.06.
Synthesis of poly(alkyl glycidyl ether)-b-poly (ε-caprolactone) (PAGE-b-PCL) copolymers
PAGE-b-PCL copolymers were prepared by ring-opening polymerization of ε-CL with PAGE as macroinitiator in the presence of Sn(Oct)2 as catalyst. 2.50 g (22 mmol) of ε-CL, 1.00 g (0.37 mmol –OH) of PAGE and 0.22 mL (0.17 mmol mL−1) of Sn(Oct)2 in xylene were charged into a flame-dried and nitrogen-purged Schlenk tube with a stirrer. The tube was purged with nitrogen and degassed several times. Then, the flask was sealed under vacuum and put into a pre-heated oil bath. The reaction was carried out at 130 °C for 24 h, and then it was stopped by removing the flask from the oil bath. Purification was performed by dissolving the reaction mixture in a small amount of CH2Cl2 and pouring it into an excess of hexane with stirring. The copolymers were collected and dried in vacuum. The results were also summarized in Table 1.PAGE3k-b-PCL6k: 1HNMR, δ (CDCl3, ppm): 1.21 (t, 3H, CH3CH2O–), 1.38 (m, 110H, –COCH2CH2CH2CH2CH2O–), 1.65 (m, 220H, –COCH2CH2CH2CH2CH2O–), 2.31 (t, 110H, –COCH2CH2CH2CH2CH2O–), 3.47–3.67 (m, 120H, –CH2CH(CH2O–)O–), 3.99 (d, 48H, –CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.10 (t, 110H, –COCH2CH2CH2CH2CH2O–), 5.20 (d, 24H, –CH
CH2), 4.10 (t, 110H, –COCH2CH2CH2CH2CH2O–), 5.20 (d, 24H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.28 (d, 24H, –CH
CH2), 5.28 (d, 24H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.91 (m, 24H, –CH
CH2), 5.91 (m, 24H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). GPC: Mn = 15
CH2). GPC: Mn = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730, Mw/Mn = 1.44.
730, Mw/Mn = 1.44.
Synthesis of poly(allyl glycidyl ether)-b-poly (ε-caprolactone)-b-polyethylene glycol (PAGE-b-PCL-b-PEG) triblock copolymers
Under nitrogen, 1.05 g (0.30 mmol) of mPEG-COOH3.5k, 74.30 mg (0.36 mmol) of dicyclohexylcarbodiimide (DCC), 52.00 mg (0.43 mmol) of N,N-dimethyl aminopyridine (DMAP), 1.50 g (0.17 mmol) of PAGE3k-b-PCL6k copolymer dissolved in 25 mL of anhydrous CH2Cl2 were added to a flame-dried and nitrogen-purged Schlenk tube equipped with a stirrer. The reaction was carried out at room temperature for 48 h. When the reaction was finished, the mixture was filtered to get the filtrate. The filtrate was precipitated with ether. Subsequently, the precipitate was dissolved into appropriate DMF and the solution was dialyzed against water using a dialysis membrane tube (Mw cutoff 5000 Da) at room temperature for 5 days and then the solution was dialyzed against acetone to remove water. Finally, acetone in the dialyzed solution was evaporated under reduced pressure and dried in vacuum. The results were summarized in Table 1.PAGE3k-b-PCL6k-b-PEG3.5k: 1HNMR, δ (CDCl3, ppm): 1.21 (t, 3H, CH3CH2O–), 1.38 (m, 110H, –COCH2CH2CH2CH2CH2O–), 1.65 (m, 220H, –COCH2CH2CH2CH2CH2O–), 2.31 (t, 110H, –COCH2CH2CH2CH2CH2O–), 2.56 (dd, 4H, –OCOCH2CH2COOH), 3.39 (s, 3H, CH3O–), 3.47–3.75 (m, 450H, –CH2CH(CH2O–)O–, –OCH2CH2O–), 3.99 (d, 48H, –CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.10 (t, 110H, –COCH2CH2CH2CH2CH2O–), 5.20 (d, 24H, –CH
CH2), 4.10 (t, 110H, –COCH2CH2CH2CH2CH2O–), 5.20 (d, 24H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.28 (d, 24H, –CH
CH2), 5.28 (d, 24H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.91 (m, 24H, –CH
CH2), 5.91 (m, 24H, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). GPC: Mn = 27
CH2). GPC: Mn = 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170, Mw/Mn = 1.30.
170, Mw/Mn = 1.30.
Synthesis of poly (allyl glycidyl ether/cysteine)-b-poly (ε-caprolactone)-b-polyethylene glycol (PAGE/cys-b-PCL-b-PEG) triblock copolymers
Zwitterionic amphiphilic triblock copolymers PAGE/cys-b-PCL-b-PEG were prepared by click reaction of cysteine onto PAGE-b-PCL-b-PEG copolymers. In a typical experiment, the solution containing 1.00 g (1.78 mmol C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) of PAGE3k-b-PCL6k-b-PEG3.5k, 2.80 g (16 mmol) of cysteine and 0.45 g (2.74 mmol) of AIBN (molar ratio: [C
C) of PAGE3k-b-PCL6k-b-PEG3.5k, 2.80 g (16 mmol) of cysteine and 0.45 g (2.74 mmol) of AIBN (molar ratio: [C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C]/[SH]/[AIBN] = 1
C]/[SH]/[AIBN] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) in 30 mL of anhydrous DMF was bubbling for 1.5 h under nitrogen flow, then the mixture was heated at 70 °C for 48 h. After that, the mixture was cooled to room temperature and DMF was evaporated under reduced pressure. The resultant products were dialyzed against water using a dialysis membrane tube (Mw cutoff 2000 Da) at room temperature for 5 days to remove the excess of cysteine and then the solution was dialyzed against acetone to remove water. At last, acetone in the dialyzed solution was evaporated under reduced pressure and dried in vacuum.
1.5) in 30 mL of anhydrous DMF was bubbling for 1.5 h under nitrogen flow, then the mixture was heated at 70 °C for 48 h. After that, the mixture was cooled to room temperature and DMF was evaporated under reduced pressure. The resultant products were dialyzed against water using a dialysis membrane tube (Mw cutoff 2000 Da) at room temperature for 5 days to remove the excess of cysteine and then the solution was dialyzed against acetone to remove water. At last, acetone in the dialyzed solution was evaporated under reduced pressure and dried in vacuum.
PAGE3k/cys-b-PCL6k-b-PEG3.5k: 1HNMR, δ (DMSO-d6, ppm): 1.21 (t, 3H, CH3CH2O–), 1.38 (m, 110H, –COCH2CH2CH2CH2CH2O–), 1.65 (m, 220H, –COCH2CH2CH2CH2CH2O–), 1.71 (t, 48H, –OCH2CH2CH2S–), 2.31 (t, 110H, –COCH2CH2CH2CH2CH2O–), 2.54 (t, 48H, –OCH2CH2CH2S–), 2.76–3.10 (m, 48H, –SCH2CH(NH2)COOH), 3.39 (s, 3H, CH3O–), 3.47–3.75 (m, 450H, –CH2CH(CH2O–)O–, –OCH2CH2O–), 4.10 (t, 110H, –COCH2CH2CH2CH2CH2O–), 4.54 (d, 24H, –SCH2CH(NH2)COOH).
Preparation of the blank and DOX-loaded micelles from the zwitterionic amphiphilic triblock copolymers PAGE/cys-b-PCL-b-PEG
The blank micelle solution of PAGE/cys-b-PCL-b-PEG triblock copolymers were prepared by the dialysis method. 150 mg of the copolymer PAGE3k/cys-b-PCL6k-b-PEG3.5k was dissolved in 3 mL of freshly prepared DMF in a 100 mL round-bottom flask with a stirrer, and 25 mL of the twice-distilled water was added dropwise (ca. one drop/25 s). Then, the solution was dialyzed against the twice-distilled water using a dialysis membrane tube (Mw cutoff 2000 Da) for 3 days to remove DMF. The polymer solution and twice-distilled water were added to a 100 mL volumetric flask to make the polymer solution of 1.50 mg mL−1. The samples were sonicated to homogenize the solution. According to the above method, 1.2 mg mL−1 of PAGE1k/cys-b-PCL3k-b-PEG2k micelle solution was achieved.The DOX-loaded micelles were also prepared by using the dialysis method. DOX·HCl (6 mg) and 3-fold molar triethylamine (TEA) were dissolved in DMF (3 mL) and kept stirring for 2 h to remove hydrochloride. After this, the copolymer PAGE3k/cys-b-PCL6k-b-PEG3.5k (60 mg) was added and stirred to form a homogeneous solution. After stirring for another 0.5 h, the solution was dialyzed against twice-distilled water using a dialysis tube (Mw cutoff, 2000 Da) for 24 h and the solution outside the tube was replaced by fresh twice-distilled water every 4 h. And then, the solution in the tube was freeze-dried to obtain the final product which was stored at −20 °C until further experiments. The DOX loading content (LC) and entrapment efficiency (EE) were determined by UV-vis spectrophotometer. 3 mg of the above product was dissolved in 5 mL of DMF. The concentration of DOX at 481 nm was recorded with reference to a calibration curve of pure DOX–DMF solution. The LC and EE of DOX were calculated using the following formulas, respectively.
In vitro release of DOX from PAGE3k/cys-b-PCL6k-b-PEG3.5k micelles
The in vitro DOX release properties from the PAGE3k/cys-b-PCL6k-b-PEG3.5k self-assembled micelles were determined as follows: 2 mg of the freeze-dried product was dissolved in 3 mL of PBS (pH = 7.4) and then the solution was placed in a dialysis membrane tube (Mw cutoff 2000 Da). The dialysis tube was then immersed in 40 mL of PBS buffer at pH 7.4, and kept in a 37 °C water bath. At specific time intervals, a 4 mL (Ve) sample was taken out and replaced by 4 mL fresh PBS to maintain the total volume. The concentration of DOX in different samples was determined using UV-vis spectrophotometer with reference to a calibration curve of pure DOX–PBS solution. The cumulative drug release percent (Er) was calculated based on the equation.where mDOX represents the amount of DOX in the micelle (mg), V0 is the whole volume of the release media (V0 = 40 mL), and Cn represents the concentration of DOX in the nth sample (mg). The in vitro experiments were repeated three times, and all samples were analyzed in triplicate to get the final release curve.
Cell proliferation and morphology30
where, Atest is the absorbance of the experiment group, Acontrol is the absorbance of the control group and Ablank is the absorbance of the blank group (without any cells). The final result was assumed to be the mean of triplicate measurements. Results were presented as the mean ± SD, and Student's t test was used to determine statistical significance. P < 0.05 was considered significant.
Results and discussion
Synthesis of zwitterionic amphiphilic triblock copolymers PAGE/cys-b-PCL-b-PEG
The zwitterionic amphiphilic triblock copolymers PAGE/cys-b-PCL-b-PEG were synthesized according to the procedure shown in Scheme 1. The copolymers PAGE-b-PCL were synthesized first by ring-opening polymerization of ε-CL with PAGE as macroinitiator and Sn(Oct)2 as catalyst. And then the PAGE-b-PCL copolymers reacted with mPEG-COOH to give the amphiphilic triblock copolymers PAGE-b-PCL-b-PEG. The double bonds in the side chains provided the possibility to react with ω-functional mercaptons via click reaction. The zwitterionic amphiphilic triblock copolymers bearing amino acid residues were obtained by click reaction of L-cysteine (–SH) and the double bonds along the PAGE-b-PCL-b-PEG.PAGE monoethyl ethers were obtained by anionic ring-opening polymerization of AGE using C2H5ONa as initiator. Fig. 1A showed the 1HNMR spectrum of PAGE3k. The degree of polymerization of PAGE was calculated from the relative integration intensities of protons of the methyl end group (–CH3) at 1.21 ppm and protons of the pendant double bonds (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) at 5.91 ppm. Mn determined from 1HNMR spectra was close to the theoretical value. It can be seen that the GPC trace of PAGE3k was narrow and monomodal (Fig. 2a), which indicated that PAGE with narrow molecular weight distribution was obtained.
CH2) at 5.91 ppm. Mn determined from 1HNMR spectra was close to the theoretical value. It can be seen that the GPC trace of PAGE3k was narrow and monomodal (Fig. 2a), which indicated that PAGE with narrow molecular weight distribution was obtained.
 | ||
| Fig. 1 1HNMR of (A) PAGE3k in CDCl3, (B) PAGE3k-b-PCL6k in CDCl3, (C) PAGE3k-b-PCL6k-b-PEG3.5k in CDCl3 and (D) PAGE3k/cys-b-PCL6k-b-PEG3.5k in DMSO-d6. | ||
The block copolymer intermediates PAGE-b-PCL were prepared by ring-opening polymerization of ε-CL using PAGE as macroinitiator in the presence of Sn(Oct)2. The length of PCL blocks was controlled by the molecular ratio of the monomer to the initiator. In this way, a series of block copolymers differing in the length of the polymer blocks were obtained as listed in Table 1. Fig. 1B showed the 1HNMR spectrum of PAGE3k-b-PCL6k. Compared to the spectrum of PAGE3k, the new peak at 1.38 ppm, 1.65 ppm, 2.31 ppm and 4.10 ppm were attributed to the methylenes of ε-CL. The peak at 5.91 ppm was attributed to (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) of pendant double bonds. The actual molar ratio of the two components of the copolymers was estimated by comparing the integrations of PCL methylene proton signals at 4.10 ppm and signals at 5.90 ppm assigned to the double bonds of the PAGE blocks. Compared to Fig. 2a, the GPC traces of the copolymers PAGE3k-b-PCL6k showed narrow and monomodal peak and shifted to the higher molecular weight region (Fig. 2b). These results indicated that the copolymerization of PAGE and ε-CL proceeded successfully.
CH2) of pendant double bonds. The actual molar ratio of the two components of the copolymers was estimated by comparing the integrations of PCL methylene proton signals at 4.10 ppm and signals at 5.90 ppm assigned to the double bonds of the PAGE blocks. Compared to Fig. 2a, the GPC traces of the copolymers PAGE3k-b-PCL6k showed narrow and monomodal peak and shifted to the higher molecular weight region (Fig. 2b). These results indicated that the copolymerization of PAGE and ε-CL proceeded successfully.
The block copolymer intermediates PAGE-b-PCL-b-PEG were prepared by condensation reaction of PAGE-b-PCL and mPEG-COOH. The compositions of the triblock copolymers were complex. Fig. 1C showed the 1HNMR spectrum of PAGE3k-b-PCL6k-b-PEG3.5k. Compared to the block copolymer PAGE3k-b-PCL6k, the peaks at 3.47–3.75 ppm in Fig. 1C became bigger and the GPC traces of the copolymers PAGE3k-b-PCL6k-b-PEG3.5k showed higher weight region (Fig. 2c). These proved that the formation of triblock copolymer PAGE3k-b-PCL6k-b-PEG3.5k.
Finally, the cysteine molecules were conjugated to the pendant double bonds of the copolymers PAGE-b-PCL-b-PEG via click reaction to give the PAGE/cys-b-PCL-b-PEG. Fig. 1D showed the 1HNMR of the final product. Compared to PAGE3k-b-PCL6k-b-PEG3.5k, the signals at 1.71 ppm (–CH2CH2CH2S–) and 2.54 ppm (–CH2CH2CH2S–) and the signals of cysteine residues at 2.74–2.90 ppm (–SCH2CH(NH2)COOH), 4.54 ppm (–SCH2CH(NH2)COOH) were observed clearly. At the same time, the signals at 5.20, 5.28 ppm (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) and 5.90 ppm (–CH
CH2) and 5.90 ppm (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), which are the feature of the pendant double bonds of PAGE block disappeared absolutely, suggesting the occurrence of click reaction between the double bonds of PAGE3k-b-PCL6k-b-PEG3.5k and –SH groups of cysteine.
CH2), which are the feature of the pendant double bonds of PAGE block disappeared absolutely, suggesting the occurrence of click reaction between the double bonds of PAGE3k-b-PCL6k-b-PEG3.5k and –SH groups of cysteine.
Self-assembly properties of the zwitterionic amphiphilic triblock copolymers PAGE/cys-b-PCL-b-PEG
The self-assembly behavior of the zwitterionic amphiphilic triblock copolymers PAGE/cys-b-PCL-b-PEG in aqueous solution was investigated by fluorescence technique using pyrene as probe (c = 6 × 10−4 mol L−1). Fig. 3 showed a plot of the pyrene fluorescence intensity ratio I373/I383 (I373, the first peak on the excitation spectra; I383, the third peak) versus the concentration of the PAGE/cys-b-PCL-b-PEG. It is well-known that the critical micelles concentration (CMC) is one of the most important parameters of assessing the self-assembly process of amphiphiles in solution, which is determined from the intersection of the two tangent lines as shown in Fig. 3. In present study, the derived CMC of PAGE3k/cys-b-PCL6k-b-PEG3.5k was 0.20 mg mL−1 and the derived CMC of PAGE1k/cys-b-PCL3k-b-PEG2k was 0.47 mg mL−1. | ||
| Fig. 3 Plot of the ratio I373/I383 (from pyrene excitation spectra at λexc = 335 nm) versus the concentration of (A) PAGE3k/cys-b-PCL6k-b-PEG3.5k and (B) PAGE1k/cys-b-PCL3k-b-PEG2k. | ||
The morphology and diameter distribution of the formed micelles were analysed by using cryo-TEM and DLS. The cryo-TEM image (Fig. 4) showed that these zwitterionic amphiphilic copolymers could self-assemble into spherical micelles. The average diameter of the micelles determined by DLS was about 26 nm (PAGE3k/cys-b-PCL6k-b-PEG3.5k) and about 19 nm (PAGE1k/cys-b-PCL3k-b-PEG2k) (Fig. 5). In addition, their poly-dispersity indexes (PDI) measured by DLS were 0.156 (PAGE3k/cys-b-PCL6k-b-PEG3.5k) and 0.112 (PAGE1k/cys-b-PCL3k-b-PEG2k). It is believed that small particles (<100 nm) with a hydrophilic surface have the greatest ability to evade the MPS.31 In fact, the micelles formed by the obtained copolymers possess both small size (<100 nm) and hydrophilic surface. Therefore, it increases the probability for the nanoparticles to reach their target for prolonged circulation time.
 | ||
| Fig. 4 Cryo-TEM images of the self-assembled micelles of PAGE/cys-b-PCL-b-PEG: (A) PAGE3k/cys-b-PCL6k-b-PEG3.5k; (B) PAGE1k/cys-b-PCL3k-b-PEG2k. | ||
 | ||
| Fig. 5 DLS of the self-assembled micelles of PAGE/cys-b-PCL-b-PEG: (A) PAGE3k/cys-b-PCL6k-b-PEG3.5k; (B) PAGE1k/cys-b-PCL3k-b-PEG2k. | ||
Fig. 6 showed a plot of the zeta potential versus time. It can be seen that the zeta potential of PAGE3k/cys-b-PCL6k-b-PEG3.5k micelles changed from −34 mV to −28 mV and the zeta potential of PAGE1k/cys-b-PCL3k-b-PEG2k micelles changed from −29 mV to −27 mV. The results revealed the change of zeta potential was not significant as time gone, suggesting these micelles from PAGE/cys-b-PCL-b-PEG were stable.
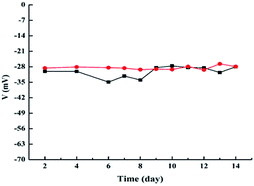 | ||
| Fig. 6 Zeta potential of the self-assembled micelles of PAGE/cys-b-PCL-b-PEG:PAGE3k/cys-b-PCL6k-b-PEG3.5k (black); PAGE1k/cys-b-PCL3k-b-PEG2k (red). | ||
 | ||
| Fig. 7 Release curve of DOX from PAGE3k/cys-b-PCL6k-b-PEG3.5k micelles at 37 °C (phosphate buffer, pH 7.4). | ||
In vitro release of DOX from PAGE3k/cys-b-PCL6k-b-PEG3.5k micelles
DOX, a hydrophobic anticancer drug, was encapsulated into PAGE3k/cys-b-PCL6k-b-PEG3.5k micelles to evaluate the drug release ability. The drug loading content of PAGE3k/cys-b-PCL6k-b-PEG3.5k micelles was determined to be 4.28% and the encapsulation efficiency of the copolymer was 40.38%. Subsequently, in vitro release of the drug from DOX-loaded micelles was conducted under simulated physiological conditions (phosphate buffer pH 7.4, 37 °C). Fig. 7 showed about 67% DOX was released from DOX-loaded micelles in first 28 h and then it slowly rose to 72% in the followed 68 h. It indicated that the encapsulated DOX was released from the PAGE3k/cys-b-PCL6k-b-PEG3.5k micelles in a controlled manner.Biocompatibility of the copolymers PAGE/cys-b-PCL-b-PEG
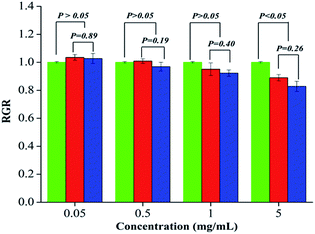
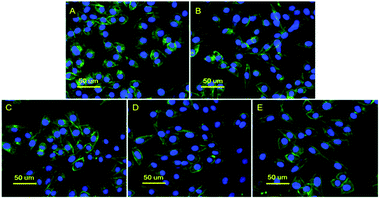
In order to test the biocompatibility of the obtained copolymers, the in vitro cytotoxicity was determined. Fig. 8 shows L929 cells proliferation in the presence of different concentrations of PAGE3k/cys-b-PCL6k-b-PEG3.5k or PAGE1k/cys-b-PCL3k-b-PEG2k assessed by MTT assay. The relative growth rate (RGR) in the MTT test was adopted to evaluate the cell toxicity at a predetermined time. After the detailed statistical analysis by SPSS 13.0, it was found that except the highest concentration (5 mg mL−1), the other experimental specimens at dilute concentrations ranging from 0.05 mg mL−1 to 1 mg mL−1 were considered to be of no cytotoxicity compared with the control group (p > 0.05, n = 6). And the statistical difference between the highest concentration (5 mg mL−1) treated group and control group is reasonably explained by the water insoluble characteristic of copolymers, which may influence the absorbance during the MTT assay. In addition, there were no statistical difference between PAGE3k/cys-b-PCL6k-b-PEG3.5k and PAGE1k/cys-b-PCL3k-b-PEG2k treated group at each concentration.The event was confirmed by F-actin staining (Fig. 9). In the experiment, FITC-phalloidin (green fluorescence) and DAPI (blue fluorescence) were used to incubate the cells. The results show that there was no significant difference in cell density after 48 h cell culture. The effects on cell morphology were not found in the presence of different concentrations of PAGE3k/cys-b-PCL6k-b-PEG3.5k or PAGE1k/cys-b-PCL3k-b-PEG2k. This result is in agreement with the previous relative growth rate (RGR) in the cytotoxicity test-ing, indicating that both of the copolymers have good biocompatibility with L929 cells at relatively lower concentrations. Therefore, these zwitterionic amphiphilic triblock copolymers are expected to act as promising nanocarriers for various drug deliveries.
Conclusions
In summary, the zwitterionic amphiphilic triblock copolymers bearing amino acid residues PAGE/cys-b-PCL-b-PEG were successfully synthesized via an easy and efficient way. These copolymers can self-assemble in aqueous solution and form spherical micelles with a small diameter. Meanwhile, the results of zeta potential displayed these micelles were stable in solution. Moreover, the in vitro cytotoxicity assay exhibited that the copolymers have good biocompatibility with L929 cells at relatively lower concentrations. In addition, the in vitro drug release profile showed that the copolymers micelles could release DOX in a controlled manner. Therefore, these copolymers have great potential to emerge as promising nanocarriers for various drug deliveries.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21271066).Notes and references
- Z. Ma, A. Haddadi, O. Molavi, A. Lavasanifar, R. Lai and J. Samuel, J. Biomed. Mater. Res., Part A, 2008, 86, 300–310 CrossRef PubMed.
- M. Prabaharan, J. J. Grailer, S. Pilla, D. A. Steeber and S. Gong, Biomaterials, 2009, 30, 3009–3019 CrossRef CAS PubMed.
- Y. Tao, R. Liu, M. Chen, C. Yang and X. Liu, J. Mater. Chem., 2012, 22, 373–380 RSC.
- M. Auffan, J. Rose, J. Y. Bottero, G. V. Lowry, J. P. Jolivet and M. R. Wiesner, Nat. Nanotechnol., 2009, 4, 634–641 CrossRef CAS PubMed.
- S. Zhai, Y. Ma, Y. Chen, D. Li, J. Cao, Y. Liu, M. Cai, X. Xie, Y. Chen and X. Luo, Polym. Chem., 2014, 5, 1285–1297 RSC.
- M. A. Dobrovolskaia, P. Aggarwal, J. B. Hall and S. E. McNeil, Mol. Pharm., 2008, 5, 487–495 CrossRef CAS PubMed.
- Y. J. Shih, Y. Chang, A. Deratani and D. Quemener, Biomacromolecules, 2012, 13, 2849–2858 CrossRef CAS PubMed.
- H. Ma, J. Hyun, P. Stiller and A. Chilkoti, Adv. Mater., 2004, 16, 338–341 CrossRef CAS.
- T. M. Blättler, S. Pasche, M. Textor and H. J. Griesser, Langmuir, 2006, 22, 5760–5769 CrossRef PubMed.
- L. Li, S. Chen, J. Zheng, B. D. Ratner and S. Jiang, J. Phys. Chem. B, 2005, 109, 2934–2941 CrossRef CAS PubMed.
- E. Ostuni, R. G. Chapman, R. E. Holmlin, S. Takayama and G. M. Whitesides, Langmuir, 2001, 17, 5605–5620 CrossRef CAS.
- Q. Liu, A. Singh, R. Lalani and L. Liu, Biomacromolecules, 2012, 13, 1086–1092 CrossRef CAS PubMed.
- E. Österberg, K. Bergström, K. Holmberg, A. J. Riggs, M. J. VanAlstine, P. T. Schuman, L. N. Burns and M. J. Harris, Colloids Surf., A, 1993, 77, 159–169 CrossRef.
- R. A. Statz, J. R. Meagher, E. A. Barron and B. P. Messersmith, J. Am. Chem. Soc., 2005, 127, 7972–7973 CrossRef PubMed.
- S. Lin, B. Zhang, M. J. Skoumal, B. Ramunno, X. Li, C. Wesdemiotis, L. Liu and L. Jia, Biomacromolecules, 2011, 12, 2573–2582 CrossRef CAS PubMed.
- W. Yang, H. Xue, W. Li, J. Zhang and S. Jiang, Langmuir, 2009, 25, 11911–11916 CrossRef CAS PubMed.
- Z. Zheng, S. Chen and S. Jiang, Biomacromolecules, 2006, 7, 3311–3315 CrossRef PubMed.
- W. Yang, S. Chen, G. Cheng, H. Vaisocherova, H. Xue, W. Li, J. Zhang and S. Jiang, Langmuir, 2008, 24, 9211–9214 CrossRef CAS PubMed.
- R. Lalani and L. Liu, Biomacromolecules, 2012, 13, 1853–1863 CrossRef CAS PubMed.
- W. Feng, S. Zhu, K. Ishihara and J. L. Brash, Langmuir, 2005, 21, 5980–5987 CrossRef CAS PubMed.
- S. Jiang and Z. Cao, Adv. Mater., 2010, 22, 920–932 CrossRef CAS PubMed.
- K. Shiraishi, T. Ohnishi and K. Sugiyama, Macromol. Chem. Phys., 1998, 199, 2023–2028 CrossRef CAS.
- J. E. Rosen and F. X. Gu, Langmuir, 2011, 27, 10507–10513 CrossRef CAS PubMed.
- Q. Shi, Y. Su, W. Chen, J. Peng, L. Nie, L. Zhang and Z. Jiang, J. Membr. Sci., 2011, 366, 398–404 CrossRef CAS.
- Q. Liu, A. Singh and L. Liu, Biomacromolecules, 2013, 14, 226–231 CrossRef CAS PubMed.
- H. Wang, S. Wang, H. Su, K. J. Chen, A. L. Armijo, W. Y. Lin, Y. Wang, J. Sun, K. Kamei, J. Czernin, C. G. Radu and H. R. Tseng, Angew. Chem., 2009, 48, 4344–4348 CrossRef CAS PubMed.
- C. Stoffelen and J. Huskens, Chem. Commun., 2013, 49, 6740–6742 RSC.
- W. Yuan, J. Yuan, F. Zhang, X. Xie and C. Pan, Macromolecules, 2007, 40, 9094–9102 CrossRef CAS.
- Z. Hu, X. Fan, H. Wang and J. Wang, Polymer, 2009, 50, 4175–4181 CrossRef CAS.
- W. Zhang, W. Jiang, D. Zhang, G. Bai, P. Lou and Z. Hu, Polym. Chem., 2015, 6, 2274–2282 RSC.
- A. Kumari, S. K. Yadav and S. C. Yadav, Colloids Surf., B, 2010, 75, 1–18 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |