Poly(4-styrenesulfonic acid-co-maleic acid)-sodium-modified magnetic reduced graphene oxide for enhanced adsorption performance toward cationic dyes†
Yu-Bei Song,
Xiao-Dong Song,
Chang-Jing Cheng* and
Zhi-Gang Zhao
College of Chemistry and Environment Protection Engineering, Southwest University for Nationalities, Chengdu, Sichuan 610041, P. R. China. E-mail: chengcj@swun.edu.cn; Fax: +86-28-8552-2315; Tel: +86-28-8552-2792
First published on 7th October 2015
Abstract
By combining the advantages of poly(4-styrenesulfonic acid-co-maleic acid) sodium (PSSMA) with abundant anionic functional groups (–COO− and –SO3−), graphene oxide (GO) with high specific surface area and Fe3O4 nanoparticles with excellent magnetic responsiveness, a novel type of PSSMA-modified magnetic reduced graphene oxide nanocomposite (PSSMA/M-rGO) was synthesized via a simple and facile one-step solvothermal method and used for removing cationic dyes from aqueous solutions in this study. The as-synthesized PSSMA/M-rGO was characterized by Fourier transform infrared spectroscopy, UV-vis spectroscopy, scanning electron microscopy, transmission electron microscopy, thermogravimetric analysis, X-ray diffraction, vibrating sample magnetometry, dynamic light scattering and nitrogen adsorption-desorption technique. Three typical cationic dyes, basic fuchsin (BF), crystal violet (CV) and methylene blue (MB) were used as model dye pollutants to evaluate the adsorption performance of the resultant PSSMA/M-rGO. The adsorption of three cationic dyes onto both PSSMA/M-rGO and M-rGO without PSSMA modification on the surface were systematically investigated at different experiment conditions. The results indicate that the binding of PSSMA on M-rGO can significantly enhance the adsorption capacities and removal efficiencies of the three dyes. This is due to the rich –COO− and –SO3− groups on PSSMA/M-rGO having strong electrostatic interactions with the positively charged dye molecules. The adsorption kinetics and isotherms of the three dyes onto both adsorbents demonstrate that the kinetics and equilibrium adsorptions can be well-described by pseudo-second-order kinetics and Langmuir model, respectively. Moreover, the PSSMA/M-rGO nanocomposites also demonstrate high removal efficiencies toward mixed dyes of BF, CV and MB. Such functional nanocomposites with high adsorption capacity, low production cost and excellent recyclability, are promising as candidate adsorbents for highly-efficient removal of cationic organic pollutants from aqueous solutions.
1. Introduction
Organic dyes have emerged as harmful pollutants in the environment because of their widespread applications in industrial manufacture of products such as textiles, leather, paper, plastics, printing and cosmetics.1 Vast amounts of dyes together with industrial effluents are released into the aquatic environment, causing severe environmental pollution and harmful hazards to humans.2 The presence of dyes in water reduces the water transparency, and weakens the light penetration and oxygen gas solubility in water, thus decreasing the photosynthetic efficiency of aquatic plants.3 Moreover, most dyes are highly toxic and can pose teratogenic, carcinogenic and mutagenic effects to aquatic life and humans even at a very low concentrations.4 Therefore, it is of vital importance to remove dye contaminants prior to their discharge into water. To date, various methods including biological oxidation,5 photocatalytic degradation,6 ion exchange,7 membrane process8 and adsorption3,9 have been widely used to dispose dye wastewaters. Among those techniques, adsorption is considered as the most efficient and versatile approach to treat dyestuff wastewaters due to its simple design, low cost, wide adaptability and easy operation.3,9 A variety of materials, such as natural zeolites,10 modified mesoporous clays,11 biomass,9 industrial/agricultural wastes,12,13 mesoporous silica or titania,14,15 polymer microspheres,16 and carbon-based materials (activated carbon,3 carbon nanotubes,17 graphene or graphene oxide (GO)18–22), have been used as adsorbents to remove dye pollutants from contaminated water. Among those materials, GO especially has attracted considerable attention for wastewater treatment due to its distinctive one-atom-thick two-dimensional structure with large surface area, high stability as well as abundant oxygen-containing functional groups on its surface, such as carboxyl, hydroxyl and epoxy groups.18–20,22 Those unique properties of GO endow its high adsorption capacity toward cationic dyes with benzene ring structure via electrostatic, hydrogen-bonding or/and π–π stacking interactions.18–20 However, the separation of GO suspensions from treated water, especially from a large volume of water, is time-consuming and expensive due to the involvement of complex filtration or/and centrifugation process,2,18–20,22 and thus limiting the practical application of GO in wastewater treatment.Through introducing magnetic nanoparticles (MNPs), typically Fe3O4 NPs, into GO sheets to impart its convenient magnetic separability is a feasible approach to solve the problem for separation.23–26 The MNPs loaded on GO can also serve as stabilizers or separators to prevent the possible aggregation of graphene sheets.23 Solvothermal and chemical co-precipitation methods are common techniques for fabricating magnetic graphene nanocomposites.23–26 However, the introduction of MNPs into GO usually leads to decrease in adsorption capacities of the magnetic graphene nanocomposites,27 due to that the active sites (–COOH and –OH) on GO sheets are preferentially occupied by MNPs or can be partially even completely reduced.23–25 One possible solution to tackle this dilemma is to modify magnetic graphene nanocomposites with organic small molecules or polymers containing rich functional groups like –COOH, –OH and –NH2, including citric acid (CA),28 xanthate,29 poly(acrylic acid) (PAA),27 chitosan (CS),4,30 cyclodextrin (CD)26,31 and CS&CD copolymers.32 The functionalized magnetic graphene nanocomposites exhibit enhanced adsorption capacity toward various organic or inorganic contaminants through electrostatic and/or chelating interactions.4,28–32 Poly(4-styrenesulfonic acid-co-maleic acid) sodium salt (PSSMA), an anionic polyelectrolyte, contains abundant carboxyl (–COO−) and sulfonic (–SO3−) groups in the molecule (Fig. S1A†). The distinctive properties of PSSMA provide strong electrostatic affinity to various cationic pollutants, such as positively charged organic dyes and heavy metal ions. In practical application, if PSSMA can be effectively bound on MNPs-loaded graphene, the obtained nanocomposites will not only enhance the adsorption capacity toward cationic dyes, but also provide excellent magnetic separability, thus the problem of time-consuming and high-cost of GO when used as adsorbents to treat dyestuff wastewaters can be effectively solved. Our recent work also indicates that the introduction of PSSMA on the surface of Fe3O4 NPs can significantly enhance the adsorption performance of the functionalized MNPs toward a cationic dye, methylene blue (MB).33 However, due to a relatively smaller specific surface area of Fe3O4 NPs for the grafting of PSSMA functional molecules (lower than 50 m2 g−1), the dye uptake of PSSMA-modified MNPs is still limited. The maximum equilibrium adsorption capacity of MB is only 52.2 mg g−1 at an initial concentration of 250 mg L−1.
In this study, Fe3O4 NPs-loaded GO nanosheets, which combines the large specific surface area of GO and the excellent magnetic responsiveness of Fe3O4 NPs, were used for the binding of PSSMA via simple and facile one-pot solvothermal method. The PSSMA-modified magnetic rGO nanocomposites (PSSMA/M-rGO) were used to remove three typical cationic dyes including basic fuchsin (BF), crystal violet (CV) and MB from model dye wastewaters, (Fig. S1B–D†). The effects of solution pH, contact time, and initial dye concentrations on the adsorption of three dyes were systematically investigated. Moreover, adsorption mechanisms of the three dyes were also discussed in detail. The results indicate that the PSSMA/M-rGO demonstrate higher adsorption capacity and improved removal efficiency as compared to that of M-rGO without PSSMA modification. The adsorption kinetics and isotherms of three dyes onto both PSSMA/M-rGO and M-rGO show that the kinetics and equilibrium adsorptions can be well-described by the pseudo-second-order kinetic and Langmuir model, respectively. Furthermore, the PSSMA/M-rGO also exhibit high removal efficiency toward mixed dyes of BF, CV and MB. The dye-loaded PSSMA/M-rGO can be easily recovered under an external magnetic field and regenerated using 2.0 wt% NaOH ethanol solutions. Such functional nanocomposites with high adsorption capacity, low production cost and excellent recyclability, show great potential as candidate adsorbents for highly-efficient removal of cationic dye pollutants from aqueous solutions.
2. Materials and experiment methods
2.1. Chemicals and materials
Graphite powders (325 mesh) were purchased from Qingdao Huatai Lubricating and Sealing Technology Co., Ltd. (Qingdao, China). Poly(4-styrenesulfonic acid-co-maleic acid, SS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA = 3
MA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) sodium salt (PSSMA, Mw = 20
1) sodium salt (PSSMA, Mw = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was obtained from Sigma-Aldrich. Ferric chloride hexahydrate (FeCl3·6H2O), potassium permanganate (KMnO4), sodium acetate trihydrate (CH3COONa·3H2O, NaAc), basic fuchsin (BF), crystal violet (CV) and methylene blue (MB) were bought from Chengdu Kelong Chemicals (Chengdu, China). All chemicals were of analytical reagent grade. Deionized water was used throughout this study.
000) was obtained from Sigma-Aldrich. Ferric chloride hexahydrate (FeCl3·6H2O), potassium permanganate (KMnO4), sodium acetate trihydrate (CH3COONa·3H2O, NaAc), basic fuchsin (BF), crystal violet (CV) and methylene blue (MB) were bought from Chengdu Kelong Chemicals (Chengdu, China). All chemicals were of analytical reagent grade. Deionized water was used throughout this study.
2.2. Preparation of GO
GO were prepared from natural graphite power based on the modified Hummers method.34 Briefly, graphite power (1.0 g) and NaNO3 (1.0 g) were mixed in 50 mL of H2SO4 (98 wt%) in a beaker at ice temperature (0–5 °C). Then KMnO4 (6.0 g) was added in the mixture slowly under stirring over 1 h. Next, the temperature of mixture was kept at 40 °C for 2 h, and 100 mL of deionized water was added slowly. Then the reaction temperature was maintained at 90 °C for 0.5 h. After that, 200 mL of deionized water and 10 mL of hydrogen peroxide (H2O2) (30%) were added, and the color of mixture changed from dark brown to brilliant yellow. The obtained dispersion was washed using HCl (37%) to remove residual metal ions, and followed by deionized water until the pH of filtrate was neutral. After drying under vacuum at room temperature, GO solid was obtained.2.3. Preparation of PSSMA/M-rGO
PSSMA/M-rGO were prepared via simple and facile one-pot solvothermal method with slight modification.23 Typically, 0.1 g of GO was dispersed in 50 mL of ethylene glycol (EG) by ultrasonication for 2 h. Then 0.25 g of FeCl3·6H2O was added and the mixture was vigorously stirred for 2 h. After that, 0.6 g of PSSMA and 0.9 g of NaAc were added under vigorous stirring for 0.5 h. The brownish yellow mixture was transferred to a Teflon-lined stainless steel autoclave and heated at 200 °C for 10 h, and then cooled to room temperature. The obtained black product was washed using deionized water for 5 times, and dried under vacuum. The PSSMA/M-rGO serials synthesized at dosage of 0, 0.1, 0.25, 0.6 and 1.0 g were denoted as M-rGO, M-rGO-010, M-rGO-025, M-rGO-060 and M-rGO-100, separately. For comparison, 0.6 g of PSSMA-modified MNPs and rGO were also prepared using the same method as the M-rGO-060 except that no GO or FeCl3·6H2O was added during the solvothermal process, and were denoted as the Fe3O4-060 and rGO-060, respectively.2.4. Characterization
The FT-IR spectra were recorded on an IR 200 spectrometer (Thermo Nicolet, USA). Field-emission scanning electron microscope (SEM) images were obtained with a JSM-7600F microscope (JEOL, Japan) at an accelerating voltage of 15 kV. The samples were prepared by mounting a drop of dispersion onto a glass sheet. Transmission electron microscope (TEM) images were taken on a JEM-2010 microscope (JEOL, Japan) at an accelerating voltage of 120 kV. The samples were prepared by mounting a drop of dispersion onto a carbon-coated copper grid. Powder X-ray diffraction (XRD) patterns were obtained on a DX-1000 diffractometer (Dandong Fangyuan Instrument Co., Ltd, China) with Cu Kα radiation operating at 40 kV and 36 mA. The thermogravimetric analysis (TGA) was performed on a Mettler TGA/SDTA851e (Switzerland) under a constant nitrogen flow at heating rate of 5 °C min−1. Magnetic property was conducted by vibrating sample magnetometer (VSM) on a Model 6000 physical property measurement system (Quantum Design, USA) at room temperature. The Brunauer–Emmett–Teller (BET) specific surface area was determined by nitrogen adsorption–desorption isotherms (Quantachrome Instruments, USA). The zeta potentials of sample dispersions were measured on a dynamic light scattering (DLS) instrument (Zetasizer Nano-ZS, Malvern Instruments, UK).2.5. Batch adsorption experiments
In this study, all the dye adsorption experiments were carried out using a serial of glass vials (30 mL) equipped with Teflon screw caps. Batch adsorption experiments were conducted to investigate the effects of solution pH, contact time, and initial dye concentrations on the adsorption of BF, CV and MB. The effect of pH on the dye adsorption was studied by adjusting solution pH in the range from 2.0 to 9.0. The pH values of dye solutions were regulated by adding negligible volume of 0.1 M HCl or NaOH. To investigate the effect of contact time on the dye adsorption and the adsorption kinetics, the initial concentrations of BF, CV and MB were fixed at 200, 150 and 125 mg L−1, respectively, and the supernatants after magnetic separation were withdrawn at certain time intervals during the adsorption. To explore the effect of initial dye concentrations on the dye adsorption and adsorption isotherms, some preliminary experiments were performed in advance to determine the suitable initial dye concentrations in this study. It was found that the removal efficiencies of three dyes were low (less than 50%) as the initial concentrations of BF, CV and MB were higher than 600, 450 and 250 mg L−1. Therefore, to obtain relative high dye removal efficiency, the dye concentrations were determined from 50 to 600 mg L−1 for BF, 50 to 450 mg L−1 for CV and 50 to 250 mg L−1 for MB, respectively. After adsorption, the dye-loaded adsorbents were magnetically separated from dye solutions, and the dye concentrations were determined on a UV spectrophotometer (TU-1950, Persee, Beijing, China) at excitation wavelengths of 543, 585 and 664 nm for BF, CV and MB, respectively. The equilibrium adsorption capacity qe (mg g−1) and removal efficiency(%) of three dyes were calculated from eqn (1) and (2).
 | (1) |
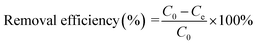 | (2) |
2.6. Desorption and regeneration
For the desorption and regeneration study, M-rGO-060 (10 mg) were added in 20 mL dye solutions with different initial concentrations (200, 150 and 125 mg L−1 for BF, CV and MB, respectively), and oscillated in a temperature-controlled shaker at 25 °C for 5 min. After magnetic separation, the dye-adsorbed M-rGO-060 were rinsed five times using NaOH ethanol solution (0.2 M), and followed by deionized water and ethanol to remove excess NaOH. The regenerated M-rGO-060 were then reused for further adsorption experiments.3. Results and discussion
3.1. Materials synthesis and characterization
Fig. 1 shows the schematic illustration for the synthesis of PSSMA/M-rGO. GO, FeCl3·6H2O and NaAc were first added in EG, and the Fe3+ would attach to the negatively charged surface of GO through electrostatic interactions and served as nucleation sites.23,24,35 After an addition of PSSMA in the above mixture, the obtained homogeneous dispersion was suffered from solvothermal reaction at 200 °C for 10 h. In this case, EG was used as both reducing reagent and solvent. NaAc alters the alkalinity and assists the reduction of GO.24 Also, in the alkaline condition, the Fe3+ in reaction solution was slowly hydrated to form Fe(OH)3, and then partially reduced by EG to form Fe(OH)2, and finally formed Fe3O4.36 As the reaction proceeds, these Fe3O4 nuclei grew into spherical particles on the partially-reduced rGO.23,24,35 Meanwhile, PSSMA functional molecules were bound on M-rGO simultaneously through a condensation reaction between the –COOH groups of PSSMA and the –OH groups of M-rGO.The chemical structures of the functionalized graphene were characterized by FT-IR. Fig. 2 shows the FT-IR spectra of GO, M-rGO, M-rGO-060 and PSSMA. For GO (Fig. 2a), the peaks at 1745, 1632, 1403 and 1057 cm−1 are respectively attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of –COOH groups, the C
O stretching vibrations of –COOH groups, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of aromatic skeletal, the C–OH deformation vibrations of phenolic groups, and the C–O–C stretching vibrations of epoxy groups.18,25–27 When Fe3O4 NPs were loaded on graphene to obtain M-rGO, the peaks of the Fe–O stretching vibrations at 580 cm−1 and the C
C stretching vibrations of aromatic skeletal, the C–OH deformation vibrations of phenolic groups, and the C–O–C stretching vibrations of epoxy groups.18,25–27 When Fe3O4 NPs were loaded on graphene to obtain M-rGO, the peaks of the Fe–O stretching vibrations at 580 cm−1 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of aromatic skeletal at 1645 cm−1 are observed. The peaks of the C
C stretching vibrations of aromatic skeletal at 1645 cm−1 are observed. The peaks of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of –COOH groups shifted to 1728 cm−1; the C–OH deformation vibrations of phenolic groups at 1404 cm−1 and the C–O–C stretching vibrations of epoxy groups at 1055 cm−1 are still observable but gradually weakened, indicating the partial reduction of the –COOH, –OH and C–O–C groups during the solvothermal process. The residual –OH groups on M-rGO may provide reactive sites for the binding of PSSMA functional molecules.23 After further functionalization of the Fe3O4 NPs-loaded GO with PSSMA, the asymmetric stretching vibrations of –SO3− groups at 1119 and 1193 cm−1, and the symmetric and asymmetric stretching vibrations of the C
O stretching vibrations of –COOH groups shifted to 1728 cm−1; the C–OH deformation vibrations of phenolic groups at 1404 cm−1 and the C–O–C stretching vibrations of epoxy groups at 1055 cm−1 are still observable but gradually weakened, indicating the partial reduction of the –COOH, –OH and C–O–C groups during the solvothermal process. The residual –OH groups on M-rGO may provide reactive sites for the binding of PSSMA functional molecules.23 After further functionalization of the Fe3O4 NPs-loaded GO with PSSMA, the asymmetric stretching vibrations of –SO3− groups at 1119 and 1193 cm−1, and the symmetric and asymmetric stretching vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in PSSMA at 1409 and 1578 cm−1 are all observed, suggesting a successful binding of PSSMA functional molecules on GO.33,36 Besides, the peaks of the C
O in PSSMA at 1409 and 1578 cm−1 are all observed, suggesting a successful binding of PSSMA functional molecules on GO.33,36 Besides, the peaks of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of –COOH groups at 1710 cm−1, and the C–O–C stretching vibrations of epoxy groups at 1055 cm−1 of GO are still observed but not as prominent as that of GO, indicating that GO was partially reduced during the solvothermal process. All above the results show that PSSMA functional molecules and Fe3O4 NPs have been successfully introduced to the partially-reduced rGO and resulted in the formation of PSSMA/M-rGO.
O stretching vibrations of –COOH groups at 1710 cm−1, and the C–O–C stretching vibrations of epoxy groups at 1055 cm−1 of GO are still observed but not as prominent as that of GO, indicating that GO was partially reduced during the solvothermal process. All above the results show that PSSMA functional molecules and Fe3O4 NPs have been successfully introduced to the partially-reduced rGO and resulted in the formation of PSSMA/M-rGO.
The reduction and surface functionalization of the Fe3O4 NPs-loaded GO were also confirmed by UV-vis in this study. Fig. S2† shows the UV-vis spectra of GO, M-rGO, M-rGO-060 and PSSMA. For GO, the representative characteristic peak at 231 nm corresponds to the π–π* electronic transitions of aromatic C–C bonds, and the shoulder at about 300 nm attributes to the n–π* transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds.37–40 After partial reduction of GO, the peak at 231 nm is red-shifted to 264 nm and no absorption peak at 300 nm is observed, indicating that the highly conjugated electronic structure is restored in M-rGO.37–41 For M-rGO-060, two new adsorption peaks at 261 and 222 nm, representing the n–π* transitions of C
O bonds.37–40 After partial reduction of GO, the peak at 231 nm is red-shifted to 264 nm and no absorption peak at 300 nm is observed, indicating that the highly conjugated electronic structure is restored in M-rGO.37–41 For M-rGO-060, two new adsorption peaks at 261 and 222 nm, representing the n–π* transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and S
O and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds and the π–π* electronic transitions of aromatic C–C bonds are also found, giving the direct evidences that PSSMA functional molecules have been successfully attached to M-rGO.
O bonds and the π–π* electronic transitions of aromatic C–C bonds are also found, giving the direct evidences that PSSMA functional molecules have been successfully attached to M-rGO.
The morphology and microstructure of M-rGO-060 were observed by SEM and TEM as shown in Fig. 3. The M-rGO-060 have crumpled and flake-like structure (Fig. 3A), and the graphene plane is sparely coated with black Fe3O4 NPs with mean diameter of about 50 nm (Fig. 3B). The Fe3O4 NPs loaded on the M-rGO-060 endow them excellent magnetic separability. Moreover, the thin layer structure of GO provides large specific surface area and abundant reactive sites for binding of PSSMA functional molecules. The rich –COOH groups in PSSMA facilitate the probability of attaching them to the Fe3O4 NPs-loaded rGO through a condensation interaction of the –COOH groups in PSSMA and the –OH groups of M-rGO, thus endowing the negatively charged surface of M-rGO-060.
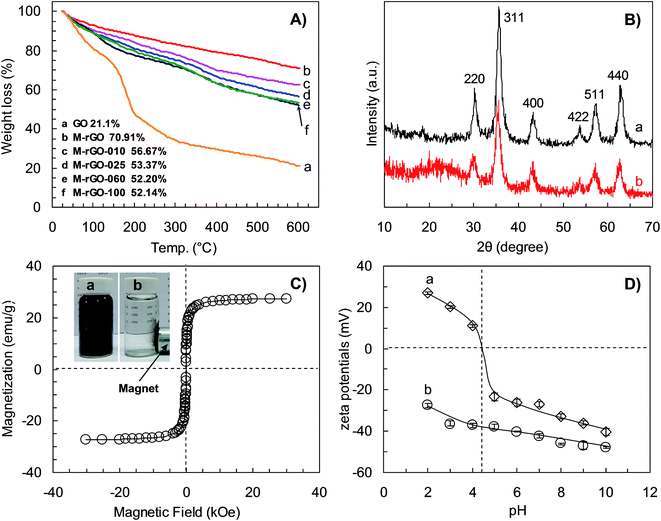
The thermal behaviors of GO and M-rGO/PSSMA series prepared with different PSSMA dosages were characterized by TGA. Fig. 4A shows the TGA curves of GO, M-rGO, M-rGO-010, M-rGO-025, M-rGO-060 and M-rGO-100. For GO, the weight loss below 120 °C is due to the evaporation of physically adsorbed water,27,35 and the weight loss from 120 °C to 300 °C is ascribed to the decomposition of oxygen-containing functional groups, such as –COOH, –OH and C–O–C.27 After the deposition of Fe3O4 NPs on graphene to obtain M-rGO, a weight loss of about 29% in the temperature range between 25 °C and 600 °C is due to the decomposition of residual –OH, –COOH and C–O–C groups.27,35 After simultaneous reduction and functionalization using PSSMA for the Fe3O4 NPs-loaded GO, the weight loss of M-rGO/PSSMA series exhibits a two-step process. The first weight loss below 120 °C is also attributable to the evaporation of physically adsorbed water on PSSMA/M-rGO,27 and the second weight loss from 120 °C to 600 °C shows the decomposition of PSSMA functional molecules covalently bound on PSSMA/M-rGO.33,36 The amount of PSSMA bound on M-rGO increase with increasing the dosage of PSSMA from 0.1 to 0.6 g during the solvothermal process, and no evident weight loss is observed for the dosage larger than 0.6 g. The binding amount of PSSMA on M-rGO-010, M-rGO-025, M-rGO-060 and M-rGO-100 are respectively 200.8, 247.4, 263.9 and 264.7 mg g−1 based on the TGA results (Fig. 4A, inset). The abundant PSSMA functional molecules on PSSMA/M-rGO play a crucial role in enhancing its uptakes toward cationic pollutants, such as cationic dyes or heavy metal ions.
XRD was employed to characterize the crystalline structure of Fe3O4 NPs on M-rGO and PSSMA/M-rGO. The XRD patterns of M-rGO and M-rGO-060 are shown in Fig. 4B, which are in accordance with the standard XRD spectra of Fe3O4 (JCPDS no. 89-4319). This further confirms the successful deposition of Fe3O4 NPs on rGO, and the binding of PSSMA functional molecules on M-rGO does not change the crystalline structure of the PSSMA/M-rGO. The magnetic responsiveness of the PSSMA/M-rGO enables them to be easily separated from dye solutions under an external magnetic field. The magnetism property of the M-rGO-060 was studied using VSM at room temperature by cycling the magnetic field between −30 kOe to 30 kOe as shown in Fig. 4C. The hysteresis and coercivity are almost undetectable, suggesting a high superparamagnetism of the M-rGO-060. The superparamagnetic property of the M-rGO-060 is critical for their practical applications, which can prevent them from occurring aggregation and make them redisperse rapidly after removing the external magnetic field (Fig. 4C, inset). The magnetic saturation value of the M-rGO-060 is 27.41 emu g−1, lower than that of Fe3O4-060 NPs (about 62.1 emu g−1). The Fe3O4-060 was synthesized using the same method as the M-rGO-060. Such high saturation magnetization allows them for effectively magnetic manipulation. Moreover, the M-rGO-060 have BET specific surface area of 426 m2 g−1, smaller than that of bare GO (the theoretically calculated value is 2630 m2 g−1), which is due to the occupation of the immobilized Fe3O4 NPs and PSSMA functional molecules on GO. However, the rich anionic functional groups (–SO3− and –COO−) on the surface and relatively large specific surface area endow them high adsorption capacities toward cationic dyes.
Surface charges of an adsorbent significantly affect its uptakes toward oppositely charged contaminants through electrostatic interactions. The pH-dependent zeta potentials of M-rGO and M-rGO-060 in aqueous solutions (0.1 mg mL−1) over a pH range of 2–10 were measured by DLS as shown in Fig. 4D. The solution pH values were adjusted by adding 0.1 M HCl or NaOH. The zeta potentials of M-rGO-060 and M-rGO decrease with increasing pH from 2.0 to 10.0. All the zeta potentials of M-rGO-060 are negative over the experimental pH range, and lower than those of M-rGO. The binding of PSSMA with rich –COO− and –SO3− functional groups on the M-rGO-060 facilitate their negatively charged surface over the investigated pH range. For M-rGO, an isoelectric point (PI) of about 4.5 is observed. Meanwhile, the M-rGO shows positively charged surface at pH lower than the PI, and negatively charged surface at pH higher than the PI. Li and co-workers also observed a similar phenomenon during the preparation of rGO nanosheets using hydrazine as the reducing reagent.38 This can be explained that the partially unreduced –COOH and –OH groups on M-rGO were protonized under an acidic condition, giving rise to positively charged surface of M-rGO. While as the pH increases, the protonized –COOH and –OH groups deprotonized gradually, therefore leading to negatively charged surface of M-rGO.
Colloidal stability of an adsorbent is also crucial for its practical application in wastewater treatment. In this study, the binding of hydrophilic PSSMA molecules on M-rGO can significantly improve the colloidal stability of PSSMA/M-rGO (Fig. S3†). As observed in Fig. S3,† the M-rGO-060 still exhibit excellent dispersion stability in water after standing for four days at room temperature (Fig. S3a–f†). Electrostatic repulsion between the –COO− and –SO3− groups on M-rGO plays a significant role in stabilizing the M-rGO-060 colloids in aqueous solution. The negatively charged surface of M-rGO-060 is also greatly beneficial to their practical applications in cationic dye wastewater treatment. However, on the other hand, due to the absence of PSSMA functional molecules on the surface, evident aggregation and precipitation are observed for the M-rGO after standing for less than 30 s (Fig. S3a′–f′†). Therefore, M-rGO exhibits limited adsorption capacity when used as an adsorbent.
3.2. Adsorption performance test
To confirm the adsorption of cationic dyes onto PSSMA/M-rGO is mainly through electrostatic interactions between the positively charged dye molecules and the negatively charged surface of PSSMA/M-rGO, the M-rGO/PSSMA serials (M-rGO, M-rGO-010, M-rGO-025, M-rGO-060 and M-rGO-100) were respectively used as adsorbents to remove three typical cationic dyes (BF, CV and MB) from aqueous solutions. As shown in Fig. 5, the adsorption capacities and removal efficiencies of three dyes onto PSSMA/M-rGO increase with increasing the PSSMA dosage, and reach a plateau as the dosage larger than 0.6 g. This indicates that the binding of PSSMA functional molecules on M-rGO plays a crucial role in enhancing the adsorption capacities of three dyes. A higher PSSMA dosage contributes to a higher binding amount of PSSMA on M-rGO during the solvothermal process, thus resulting in larger amount of negative charges on PSSMA/M-rGO for adsorption of dye molecules through strong electrostatic interactions, and finally giving rise to higher dye adsorption uptakes.Moreover, due to larger specific surface area of M-rGO compared with that of Fe3O4 NPs, larger amount of PSSMA can be bound on M-rGO. The binding amount of PSSMA on M-rGO-010 and Fe3O4-010 are 200.8 and 99.7 mg g−1, respectively. Therefore, the M-rGO/PSSMA series are expected to possess higher dye uptakes. To verify our hypothesis, Fe3O4-060, synthesized using the same method as the M-rGO-060 except that no GO was added during the solvothermal process, was also used to adsorb three cationic dyes. As observed in Fig. S4,† the Fe3O4-060 demonstrates very lower adsorption capacities and removal efficiencies toward three dyes than those of the M-rGO-060. The relatively smaller specific surface area of the Fe3O4 NPs compared with that of the M-rGO, providing relatively lower binding amount of PSSMA on the surface, may be responsible for this phenomenon. However, due to the larger specific surface area of GO for the binding of PSSMA, 0.6 g PSSMA-modified GO (rGO-060) was also prepared using the same method as the M-rGO-060 expect that no iron source (FeCl3·6H2O) was added in this study. The as-prepared rGO-060 shows higher adsorption capacities and removal efficiencies toward three dyes than those of the M-rGO-060 (Fig. S4†) due to larger binding amount of PSSMA. The FT-IR spectrum of the rGO-060 also confirms the successful attachment of PSSMA to GO (Fig. S5†). The binding amount of PSSMA on GO is about 350 mg g−1 as calculated from the TGA results. However, the separation of dye-loaded rGO-060 usually involves high-speed centrifugation or/and complex filtration process, which is usually time-consuming and uneconomic. Therefore, the practical applications of rGO-060 in dye wastewater treatment are limited. The deposition of Fe3O4 NPs on GO will inevitably occupy some active sites for the binding of PSSMA functional molecules, thus resulting in decrease of dye uptakes.23 However, considering the relatively high adsorption capacities and excellent magnetic separability of the M-rGO-060 (TOC and Fig. 4C, insets), M-rGO-060 were used to study the adsorption performance of three cationic dyes in the following investigation.
For M-rGO adsorbent, due to the absence of PSSMA functional molecules on the surface, available active sites for dye adsorption are extremely limited and therefore, dye uptakes onto M-rGO are much lower than those of M-rGO-060. π–π Stacking interactions between dye molecules and the aromatic rings of M-rGO may have contributed to the adsorption of three dyes onto M-rGO.23,27 Additionally, the uptakes of three dyes onto M-rGO-060 follow the order: BF > CV > MB. The different initial dye concentrations and interactions may account for this phenomenon. The initial concentrations of BF, CV and MB are respectively 200, 150 and 125 mg L−1. Higher initial dye concentrations provide higher driving force to overcome the mass transfer resistance of dye molecules between the aqueous phase and the solid phase, resulting in more collision between dye molecules and adsorption sites (COO− and SO3− groups) on M-rGO-060.20,23 Moreover, the difference in interactions existed between dye molecules and M-rGO-060 also contributes to the low dye uptakes onto M-rGO. For BF adsorption, except for the dominant strong electrostatic interactions between BF molecules and the –COO− and –SO3− groups on M-rGO-060, the hydrogen-bonding interactions between the –NH2 groups of BF and the –COOH and –SO3H groups on M-rGO-060, and π–π stacking interactions between BF and the aromatic rings of graphene on M-rGO-060 also exist, thus giving rise to higher dye uptakes. For CV adsorption, apart from the electrostatic interactions between CV molecules and the –COO− and –SO3− groups on M-rGO-060, π–π stacking interactions between CV and the aromatic rings of graphene also cause dye adsorption. However, for MB adsorption, only electrostatic interactions between MB molecules and the –COO− and –SO3− groups on M-rGO-060 exist. Since the adsorption capacities and removal efficiencies of three cationic dyes onto both M-rGO-060 and M-rGO are almost unchanged at pH larger than 5.0, the solution pH values were fixed at 7.0 in the subsequent adsorption study.
To well-understand the adsorption mechanisms of three cationic dyes onto both M-rGO-060 and M-rGO, three well-known kinetic models (the Lagergren pseudo first-order,44 Lagergren pseudo-second-order45 and Webber–Morris intraparticle diffusion46) were used to study the adsorption kinetics.
The Lagergren pseudo-first-order kinetic model describes the adsorption of liquid–solid system based on solid capacity,44 which can be expressed as follows:
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qt − k1t qt − k1t
| (3) |
The Lagergren pseudo-second-order kinetic model consists of all the steps of adsorption including external film diffusion, adsorption, and internal particle diffusion, which can be expressed as follows:45
 | (4) |
The Webber–Morris intraparticle diffusion model describes the adsorption process for adsorbate transport from the solution phase to the surface of an adsorbent occurs in several steps.46 The overall adsorption process may be controlled by any one of several steps, e.g. film or external diffusion, surface diffusion, pore diffusion and adsorption on the pore surface, or a combination of several steps. It can be expressed by the following equation:
| qt = kidt0.5 + Ci | (5) |
By regressing the kinetic data using above the three models, the kinetic parameters and the correlation coefficients (R2) were calculated and listed in Table 1. From the R2 values shown in Table 1, the pseudo-second-order model fits the adsorption kinetics much better than the other two models. Moreover, the qe values calculated (qe,cal) from the pseudo-second-order model are more consistent with the experimental qe values (qe,exp) than those calculated from the pseudo-first-order model, indicating that the adsorption kinetics of BF, CV and MB onto both M-rGO-060 and M-rGO follow the pseudo-second-order model. Moreover, the equilibrium adsorption capacities of M-rGO-060 for each kind of investigated dyes are nearly three times larger than those of M-rGO. This further indicates that the binding of PSSMA functional molecules on M-rGO can dramatically enhance the adsorption capacities of cationic dyes through strong electrostatic interactions between dye molecules and anionic functional groups on the adsorbent.
| Adsorbent | Dye | qe,exp (mg g−1) | Pseudo-first-order | Pseudo-second-order | Intraparticle diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 | qe,cal (mg g−1) | R2 | k2 | qe,cal (mg g−1) | R2 | kid | Ci | R2 | |||
| M-rGO-060 | BF | 388.2 | 0.0682 | 7.3 | 0.6005 | 0.038 | 392.4 | 0.9999 | 1.307 | 384.3 | 0.7854 |
| CV | 295.5 | 0.0836 | 1.7 | 0.7426 | 0.262 | 295.5 | 0.9999 | 0.245 | 294.8 | 0.3450 | |
| MB | 241.5 | 0.0474 | 1.6 | 0.4796 | 0.289 | 242.2 | 0.9999 | 0.254 | 240.9 | 0.3272 | |
| M-rGO | BF | 161.2 | 0.1587 | 48.0 | 0.8228 | 0.007 | 175.4 | 0.9989 | 9.322 | 125.3 | 0.5724 |
| CV | 117.5 | 0.0334 | 3.7 | 0.4584 | 0.336 | 122.0 | 0.9999 | 0.535 | 119.4 | 0.2628 | |
| MB | 90.5 | 0.0456 | 3.9 | 0.6838 | 0.073 | 92.6 | 0.9995 | 0.626 | 88.7 | 0.3313 | |
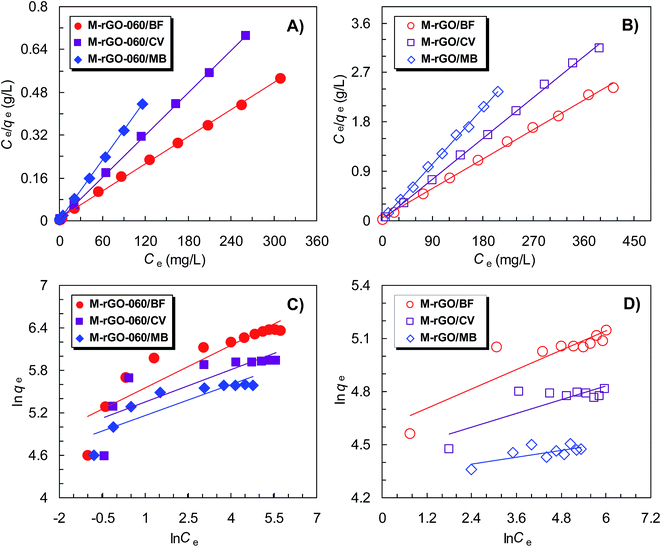
Equilibrium adsorption isotherm is one of the most important parameters that describe the relationship between the amount of adsorbate uptaken by the adsorbent and the adsorbate concentration remaining in solution. By determining the adsorption capacity of an adsorbent and modeling of isotherms by different equilibrium models, an insight into both the adsorption mechanism and the adsorbent affinity can be elucidated.12,23,42,47 Fig. 9 shows the equilibrium adsorption isotherms of three dyes onto M-rGO-060 and M-rGO. The adsorption capacities of BF, CV and MB onto both two adsorbents increase dramatically at first, suggesting a high driving force for three dyes adsorption.23 Then, the amount of adsorbed dyes reach a plateau at high equilibrium solution concentrations, reflecting the saturated dye adsorption.18,23 Moreover, after the binding of PSSMA functional molecules on M-rGO, the maximal equilibrium dye uptakes (qe,max) are raised from 171.6 to 589.5 mg g−1 for BF, from 123.4 to 379.8 mg g−1 for CV, and from 87.9 to 269.0 mg g−1 for MB, enhancing 3.43, 3.08 and 3.06 times, respectively. Those results indicate that the obvious enhancement of dye uptakes is again ascribed to the binding of PSSMA on M-rGO, which provides the strong electrostatic interactions between cationic dyes and negatively charged surface of the M-rGO-060.
 | ||
| Fig. 9 Adsorption isotherms of BF, CV and MB onto M-rGO-060 (solid) and M-rGO (hollow). Condition: pH = 7.0, m/V = 10 mg/20 mL, T = 25 °C and contact time = 5 min. | ||
To further study the adsorption mechanisms of three cationic dyes onto M-rGO-060 and M-rGO, the obtained equilibrium adsorption data are fitted by the Langmuir and Freundlich isothermal models,48–50 respectively. The Langmuir isotherm model is based on monolayer adsorption with uniform energies of adsorption on the surface,48 while the Freundlich isotherm model is based multilayer adsorption with the adsorption energy decreases with the surface coverage,49 which can be expressed as follows:
 | (6) |
 | (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus ln
qe versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce.
Ce.
Fig. 10 shows the linear plots fitting by the above models for three dyes adsorption onto M-rGO-060 and M-rGO, with their relevant parameters shown in Table 2. The linear correlation coefficients (R2) for both two adsorbents obtained from the Langmuir model are much higher than those from the Freundlich model (>99%). This indicates that the adsorption isotherms of three dyes onto both two adsorbents meet Langmuir model better than Freundlich model and assume a monolayer adsorption process. The maximum Langmuir monolayer adsorption capacities (qm) of BF, CV and MB calculated from the Langmuir model are 558.2, 384.6 and 270.3 mg g−1 for M-rGO-060, and 169.5, 120.5, and 88.5 mg g−1 for M-rGO, respectively, which are all consistent with the experimental results well. Furthermore, the adsorption process of three dyes onto both two adsorbents was also characterized by Vermeulan criteria associated with the Langmuir isotherm. The Vermeulan criteria can be expressed by a dimensionless constant separation factor (RL) given as followings:4
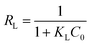 | (8) |
| Langmuir isotherm model | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dye | M-rGO-060 | M-rGO | ||||||
| qm (mg g−1) | KL (L mg−1) | R2 | RL | qm (mg g−1) | KL (L mg−1) | R2 | RL | |
| BF | 588.2 | 0.2152 | 0.9988 | 0.085–0.008 | 169.5 | 0.1329 | 0.9968 | 0.131–0.015 |
| CV | 384.6 | 0.6667 | 0.9999 | 0.030–0.003 | 120.5 | 0.5287 | 0.9988 | 0.036–0.004 |
| MB | 270.3 | 1.480 | 0.9999 | 0.013–0.003 | 88.50 | 0.6975 | 0.9984 | 0.028–0.003 |
| Freundlich isotherm model | ||||||
|---|---|---|---|---|---|---|
| Dyes | M-rGO-060 | M-rGO | ||||
| nF | KF ((mg1−1/n L1/n) g−1) | R2 | nF | KF ((mg1−1/n L1/n) g−1) | R2 | |
| BF | 4.96 | 210.3 | 0.8279 | 11.1 | 99.32 | 0.8031 |
| CV | 6.60 | 182.0 | 0.6662 | 15.4 | 84.94 | 0.6721 |
| MB | 6.91 | 151.3 | 0.7800 | 30.9 | 74.70 | 0.4980 |
The value of RL shows the adsorption process to be either unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1) or irreversible (RL = 0). In this study, all the RL values lie in between 0 and 1, indicating that the adsorption of three dyes onto both M-rGO-060 and M-rGO are favorable processes. Moreover, the RL value of the M-rGO-060 for each dye is smaller than that of the M-rGO, implying more favorable adsorption after the binding of PSSMA functional molecules on M-rGO. The favorability for three dyes onto M-rGO-060 and M-rGO follows the order: BF > CV > MB according to the RL results, which is fully in accordance with the actual adsorption experiment based on the adsorption capacities as mentioned above.
4. Conclusion
In summary, a novel type of PSSMA-modified magnetic rGO nanocomposites (PSSMA/M-rGO) have been successfully prepared via simple one-pot solvothermal method and used for removal of three typical cationic dyes (BF, CV and MB) from aqueous solutions. The resultant PSSMA/M-rGO demonstrate much higher adsorption capacities and removal efficiencies toward three cationic dyes than those of M-rGO without PSSMA modification, which is due to the strong electrostatic interactions between the positively charged dye molecules and the negatively charged surface of PSSMA/M-rGO. The adsorption of BF, CV and MB onto M-rGO-060 follows the Langmuir isotherm model with maximum monolayer adsorption capacities of 588.2 mg g−1 for BF, 384.6 mg g−1 for CV and 270.3 mg g−1 for MB, which are three times larger than those of M-rGO. The kinetics of the adsorption process fit the Lagergren pseudo-second-order kinetic model. Moreover, the M-rGO-060 also exhibit high removal efficiencies toward mixed dyes of BF, CV and MB. The saturatedly-adsorbed M-rGO-060 can be easily recovered under an external magnetic field and effectively regenerated using NaOH ethanol solution. Furthermore, after regeneration for 5 times, the M-rGO-060 still show high adsorption performances toward BF, CV and MB (>80%). The PSSMA/M-rGO developed in this work are expected to be promising adsorbents for highly-efficient removal of cationic organic pollutants from aqueous solutions, due to their high separation efficiency, low production cost and excellent recyclable property.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21106116), the project of Science & Technology department of Sichuan Province (2014GZ0012) and the project of postgraduate degree construction, Southwest University for Nationalities (2015XWD-S0703).Notes and references
- Y.-Q. Hu, T. Guo, X.-S. Ye, Q. Li, M. Guo, H.-N. Liu and Z.-J. Wu, Chem. Eng. J., 2013, 228, 392–397 CrossRef CAS PubMed.
- Z. Dong, D. Wang, X. Liu, X. Pei, L. Chen and J. Jin, J. Mater. Chem. A, 2014, 2, 5034–5040 CAS.
- E. N. E. Qada, S. J. Allen and G. M. Walker, Chem. Eng. J., 2008, 135, 174–184 CrossRef PubMed.
- L. Fan, C. Luo, X. Li, F. Lu, H. Qiu and M. Sun, J. Hazard. Mater., 2012, 215–216, 272–279 CrossRef CAS PubMed.
- S. M. Ghoreishi and R. Haghighi, Chem. Eng. J., 2003, 95, 163–169 CrossRef CAS.
- C. H. Wu, Y. Z. Zhang, S. Li, H. J. Zheng, H. Wang, J. B. Liu, K. W. Li and H. Yan, Chem. Eng. J., 2011, 178, 468–474 CrossRef CAS PubMed.
- S. Raghu and C. Ahmed Basha, J. Hazard. Mater., 2007, 149, 324–330 CrossRef CAS PubMed.
- S. Chakraborty, M. K. Purkait, S. DasGupta, S. de and J. K. Basu, Sep. Purif. Technol., 2003, 31, 141–151 CrossRef CAS.
- M. Rafatullah, O. Sulaiman, R. Hashim and A. Ahmad, J. Hazard. Mater., 2010, 177, 70–80 CrossRef CAS PubMed.
- M. Auta and B. H. Hameed, Chem. Eng. J., 2012, 198–199, 219–227 CrossRef CAS PubMed.
- R. Han, J. Zhang, P. Han, Y. Wang, Z. Zhao and M. Tang, Chem. Eng. J., 2009, 145, 496–504 CrossRef CAS PubMed.
- B. Royer, N. F. Cardoso, E. C. Lima, J. C. P. Vaghetti, N. M. Simon, T. Calvete and R. C. Veses, J. Hazard. Mater., 2009, 164, 1213–1222 CrossRef CAS PubMed.
- B. H. Hameed and A. A. Ahmad, J. Hazard. Mater., 2009, 164, 870–875 CrossRef CAS PubMed.
- C. H. Huang, K. P. Chang, H. D. Ou, Y. C. Chiang and C. F. Wang, Microporous Mesoporous Mater., 2011, 141, 102–109 CrossRef CAS PubMed.
- A. Mills, M. Sheik, C. ÓRourke and M. McFarlane, Appl. Catal., B, 2009, 89, 189–195 CrossRef CAS PubMed.
- J. Fu, Z. Chen, M. Wang, S. Liu, J. Zhang, J. Zhang, R. Han and Q. Xu, Chem. Eng. J., 2015, 259, 53–61 CrossRef CAS PubMed.
- S. Wang, C. W. Ng, W. Wang, Q. Li and Z. Hao, Chem. Eng. J., 2012, 197, 34–40 CrossRef CAS PubMed.
- H. Yan, X. Tao, Z. Yang, K. Li, H. Yang, A. Li and R. Cheng, J. Hazard. Mater., 2014, 268, 191–198 CrossRef CAS PubMed.
- G. K. Ramesha, A. Vijaya Kumara, H. B. Muralidhara and S. Sampath, J. Colloid Interface Sci., 2011, 361, 270–277 CrossRef CAS PubMed.
- P. Sharma and M. R. Das, J. Chem. Eng. Data, 2013, 58, 151–158 CrossRef CAS.
- T. Liu, Y. Li, Q. Du, J. Sun, Y. Jiao, G. Yang, Z. Wang, Y. Xia, W. Zhang, K. Wang, H. Zhu and D. Wu, Colloids Surf., B, 2012, 90, 197–203 CrossRef CAS PubMed.
- S.-T. Yang, S. Chen, Y. Chang, A. Cao, Y. Liu and H. Wang, J. Colloid Interface Sci., 2011, 359, 24–29 CrossRef CAS PubMed.
- L. Ai, C. Zhang and Z. Chen, J. Hazard. Mater., 2011, 192, 1515–1524 CrossRef CAS PubMed.
- S. Bai, X. Shen, X. Zhong, Y. Liu, G. Zhu, X. Xu and K. Chen, Carbon, 2012, 50, 2337–2346 CrossRef CAS PubMed.
- Y. Yao, S. Miao, S. Liu, L. P. Ma, H. Sun and S. Wang, Chem. Eng. J., 2012, 184, 326–332 CrossRef CAS PubMed.
- X. Liu, L. Yan, W. Yin, L. Zhou, G. Tian, J. Shi, Z. Yang, D. Xiao, Z. Gu and Y. Zhao, J. Mater. Chem. A, 2014, 2, 12296–12303 CAS.
- J. Zhang, M. S. Azam, C. Shi, J. Huang, B. Yan, Q. Liu and H. Zeng, RSC Adv., 2015, 5, 32272–32282 RSC.
- M. Namvari and H. Namaz, Polym. Int., 2014, 63, 1881–1888 CrossRef CAS PubMed.
- L. Cui, X. Guo, Q. Wei, Y. Wang, L. Gao, L. Yan, T. Yan and B. Du, J. Colloid Interface Sci., 2015, 439, 112–120 CrossRef CAS PubMed.
- L. Fan, C. Luo, M. Sun, X. Li, F. Lu and H. Qiu, Bioresour. Technol., 2012, 114, 703–706 CrossRef CAS PubMed.
- D. Wang, L. Liu, X. Jiang, J. Yu and X. Chen, Colloids Surf., A, 2015, 466, 166–173 CrossRef CAS PubMed.
- L. Fan, C. Luo, M. Sun, H. Qiu and X. Li, Colloids Surf., B, 2013, 103, 601–607 CrossRef CAS PubMed.
- Y.-B. Song, S.-N. Lv, C.-J. Cheng, G.-L. Ni, X.-W. Xie, W. Huang and Z.-G. Zhao, Appl. Surf. Sci., 2015, 324, 854–863 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- K.-F. Zhou, Y.-H. Zhu, X.-L. Yang and C.-Z. Li, New J. Chem., 2010, 34, 2950–2955 RSC.
- J. Gao, X. Ran, C. Shi, H. Cheng, T. Cheng and Y. Su, Nanoscale, 2013, 5, 7026–7033 RSC.
- J. I. Paredes, S. Villar-Rodil, A. Martínez-Alonso and J. M. D. Tascón, Langmuir, 2008, 24, 10560–10564 CrossRef CAS PubMed.
- D. Li, M. B. Müller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- S. Villar-Rodil, J. I. Paredes, A. Martínez-Alonso and J. M. D. Tascón, J. Mater. Chem., 2009, 19, 3591–3593 RSC.
- H. Peng, L. Meng, L. Niu and Q. Lu, J. Phys. Chem. C, 2012, 116, 16294–16299 CAS.
- F. He, J. Fan, D. Ma, L. Zhang, C. Leung and H. L. Chan, Carbon, 2010, 48, 3139–3144 CrossRef CAS PubMed.
- L. Ai, C. Zhang, F. Liao, Y. Wang, M. Li, L. Meng and J. Jiang, J. Hazard. Mater., 2011, 198, 282–290 CrossRef CAS PubMed.
- H. Yan, H. Li, H. Yang, A. Li and R. Cheng, Chem. Eng. J., 2013, 223, 402–411 CrossRef CAS PubMed.
- S. Largegren, K. Sven. Vetenskapsakad. Handl., 1898, 24, 1–39 Search PubMed.
- Y. S. Ho and G. M. Mckay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- W. J. Weber and J. C. Morris, J. Sanit. Eng. Div., Am. Soc. Civ. Eng., 1963, 89, 31–59 Search PubMed.
- B. S. Inbaraj and B. H. Chen, Bioresour. Technol., 2011, 102, 8868–8876 CrossRef PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1916, 38, 2221–2295 CrossRef CAS.
- H. M. F. Freundlich, J. Phys. Chem., 1906, 57, 385–471 CAS.
- G. McKay, M. El Guendi and M. Nassar, Water Res., 1987, 21, 1513–1520 CrossRef CAS.
- T. Yao, S. Guo, C. Zeng, C. Wang and L. Zhang, J. Hazard. Mater., 2015, 292, 90–97 CrossRef CAS PubMed.
- H. Zhang, X. Li, G. He, J. Zhan and D. Liu, Ind. Eng. Chem. Res., 2013, 52, 16902–16910 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Molecular structures of PSSMA and three cationic dyes: basic fuchsin (BF), crystal violet (CV) and methylene blue (MB) (Fig. S1); UV-vis spectra of GO, M-rGO, M-rGO-060 and PSSMA dispersed in water (Fig. S2); digital photographs of M-rGO-060 and M-rGO aqueous dispersions upon storing different time at room temperature (Fig. S3); adsorption capacities and removal efficiencies of BF, CV and MB onto Fe3O4-060, M-rGO-060 and rGO-060 and FT-IR spectrum of rGO-060. See DOI: 10.1039/c5ra18255g |
| This journal is © The Royal Society of Chemistry 2015 |