Nitrogen-doped carbon dots as fluorescent probe for detection of curcumin based on the inner filter effect†
Abstract
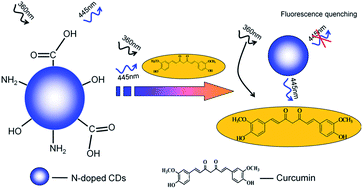
A facile, economical and green one-step hydrothermal method for N-doped CDs is presented by using citric acid as carbon source and urea as nitrogen source. The as-prepared N-doped CDs possess an average size of 5.23 nm and exhibit a high fluorescent quantum yield of 25.4%, good water solubility, and excellent photostability. The fluorescence of N-doped CDs may be quenched dramatically from curcumin via the inner filter effect. The sensitive detection method of curcumin in aqueous solution was developed with the linear range of 2.0 × 10−7 to 1.0 × 10−5 mol L−1 and detection limit of 8.48 × 10−8 mol L−1 (S/N = 3). The other common ions and related compounds could not interfere with the detection of curcumin enabling good selectivity. The proposed method was successfully applied to the determination of curcumin in urine samples and the recoveries were 95.71–103.81%.

 Please wait while we load your content...
Please wait while we load your content...