Seed-assisted, solvent-free synthesis of a CHA-type aluminophosphate molecular sieve†
Xinhong Zhao*,
Jiangbo Zhao,
Xiangping Gao and
Yu Zhao
School of Petrochemical Engineering, Lanzhou University of Technology, Lanzhou 730050, China. E-mail: Licpzhaoxh@lut.cn; Tel: +86-931-7823126
First published on 2nd November 2015
Abstract
Triclinic AlPO4-34 with CHA topology has been successfully synthesized under seed-assisted, solvent-free conditions. The synthetic conditions of this material were refined. The resultant CHA molecular sieves were characterized by PXRD, SEM, TG-DTA, CHN elements analysis, liquid 13C NMR and N2 physisorption. The results indicate that the CHA molecular sieve has the composition (Al6P6O24)(C4H9NO)2, for which morpholine acts as the structure-directing agent. The high surface area and micropore volume for the calcined CHA material imply that it can potentially be used in zeolite-related applications.
1. Introduction
Triclinic AlPO4-34 zeolite is one of the most important zeolites of the aluminophosphate family.1 It possesses a CHA structure and can be regarded as a model material of SAPO-34, which is the preferred catalyst for the conversion of methanol to olefin (MTO)2–4 due to the small pore size and moderate acid strength. It is worthwhile to note that the synthesis of the zeolites usually requires the presence of solvents such as water, alcohol or ionic liquids (ILs), which results in several issues such as low utilization efficiency of autoclaves, low product yield, and serious pollution from wastewater. Although a variety of advanced technologies, such as microwave-assisted synthesis,5–7 aminothermal synthesis2 and fluorine-free synthesis,8 have been successfully developed and used to the preparation of CHA-type molecular sieve, above problems are still not well resolved. Recently, a solvent-free route for the synthesis of zeolites was reported,9–12 in which water as solvent could be completely avoided. This route not only effectively increased the zeolite yield but also greatly reduced the production of wastes. Morris et al., also highlighted the importance of solvent-free synthesis,13 however the dosage of organic structure-directing agent in these reports was relatively high compared to the conventional hydrothermal synthesis. Therefore, it is still a challenge to synthesize aluminosilicate or aluminophosphate zeolites in a cost-saving and environmentally benign way.In the current work, we report the seed-assisted, solvent-free synthesis of triclinic CHA-type AlPO4-34 molecular sieve. The synthetic conditions of this material are optimized. The composition and pore texture of the resulting products are analyzed by various techniques.
2. Experiment
2.1 Synthesis of CHA-type aluminophosphate molecular sieve
General synthesis procedure of CHA structure was as follows: 2.656 g of 1-butyl-3-methylimidazolium bromide ([bmim]Br, Lanzhou Kaite trade Co. Ltd), 0.531 g of morpholine (Morp, Tianjin Kaixin Chemical Industry Co. Ltd), 2.478 g of aluminum isopropoxide (Sinopharm Chemical Reagent Co. Ltd), 1.384 g of phosphoric acid (85 wt% in water, Yantai Shuangshuang Chemical Reagent Co. Ltd), 0.300 g of hydrofluoric acid (40 wt% in water, Sinopharm Chemical Reagent Co. Ltd) and 0.095 g of uncalcined seed crystals if required were measured out in the molar ratio of 1Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P2O5
1P2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1HF
1HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1Morp
1Morp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2[bmim]Br and ground in a mortar for 20 min. The resulting quasi-solid mixture was transferred to 100 mL Teflon-lined stainless-steel autoclaves and crystallized under static conditions at designated temperature for 6–48 h. After crystallization, the solid product was recovered by centrifugation, washed with acetone (Beijing Chemical Plant) and distilled water, dried in air at 90 °C. The structure-directing agents occluded within the as-made samples were removed by calcination at 550 °C for 4 h. Detailed synthesis conditions were summarized in Table 1.
2[bmim]Br and ground in a mortar for 20 min. The resulting quasi-solid mixture was transferred to 100 mL Teflon-lined stainless-steel autoclaves and crystallized under static conditions at designated temperature for 6–48 h. After crystallization, the solid product was recovered by centrifugation, washed with acetone (Beijing Chemical Plant) and distilled water, dried in air at 90 °C. The structure-directing agents occluded within the as-made samples were removed by calcination at 550 °C for 4 h. Detailed synthesis conditions were summarized in Table 1.
| Samplea | Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2O5 P2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HF HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Morp Morp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ILs (molar ratio) ILs (molar ratio) |
Temperature (°C) | Time (h) | Product phasesc |
|---|---|---|---|---|
| a For all samples, the initial amount of aluminum isopropoxide used in the synthesis was fixed at 2.478 g.b 0.095 g of the as-synthesized sample T190-24h was used as the seed crystals for the last two synthesis.c Only major phase was listed. | ||||
| T150-24h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
150 | 24 | CHA |
| T150-48h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
150 | 48 | LTA |
| T170-24h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
170 | 24 | LTA |
| T170-48h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
170 | 48 | LTA |
| T190-24h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
190 | 6 | LTA |
| T190-6h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
190 | 12 | LTA |
| T190-24h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
190 | 24 | CHA |
| T190-48h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
190 | 48 | CHA |
| Seed0-IL0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
190 | 24 | CHA + prephase |
| Seed5-IL0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0b 0b |
190 | 24 | CHA |
| Seed5-IL0-Morp0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0b 0b |
190 | 24 | — |
2.2 Characterization
Powder X-ray powder diffraction (PXRD) patterns of the synthesized materials were conducted on a D/Max-2400 Rigaku diffractometer with Cu Kα radiation operated at 40 kV and 150 mA. Scanning electron microscope images were obtained from JSM-6701F instruments with Energy Dispersive Spectrometer (EDS). CHN analysis was carried out on an Elemental Vario EL analyzer. Thermogravimetric (TG) and differential thermal analysis (DTA) (Netzsch, STA409) was performed in air at a heating rate of 10 °C min−1. The solution NMR experiment was performed on an Agilent Technologies 600 MHz PremiumCompact+ Spectrometer. The as-synthesized sample was dissolved in a 36% DCl solution at room temperature. Nitrogen adsorption studies were conducted on a Micromeritics ASAP 2020 surface area and pore size analyzer at −196 °C. Samples were outgassed at 200 °C for 4 h prior to measurements. Specific surface areas of materials were calculated from the adsorption data obtained at p/p0 between 0.06 and 0.20, using the Langmuir equation.3. Results and discussion
3.1 Optimization of the synthesis
In our previous research, we found that CHA-type AlPO4-34 aluminophosphate molecular sieve could be obtained in the following initial composition 1Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P2O5
1P2O5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1HF
1HF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1morpholine
1morpholine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2[bmim]Br. However, this synthesis used expensive ionic liquids, and the product contained minor impurity-a layered prephase (a layered crystalline precursor phase associated with CHA).14,15 In the current work, the crystallization conditions of AlPO4-34 molecular sieve were further optimized to obtain pure CHA phase in a cost-saving way.
2[bmim]Br. However, this synthesis used expensive ionic liquids, and the product contained minor impurity-a layered prephase (a layered crystalline precursor phase associated with CHA).14,15 In the current work, the crystallization conditions of AlPO4-34 molecular sieve were further optimized to obtain pure CHA phase in a cost-saving way.
It is well known that crystallization temperature and time are two key factors in controlling the purity and crystallinity of the zeolite phase. Fig. 1 illustrates the XRD patterns of eight samples synthesized under different temperature and time. The XRD patterns of T150-24h, T190-24h and T190-48h demonstrate that these three samples are aluminophosphate molecular sieve with CHA topology, which are in agreement with the simulated CHA structure,16 while the other five samples (T150-48h, T170-24h, T170-48h, T190-6h and T190-12h) are pure LTA structure. For the sample T150-24h a weak diffraction peak at 2 theta = 6.34° can be attributed to the prephase. These results suggest that both high crystallization temperature, short crystallization time, and the opposite conditions (i.e. low temperature and long crystallization time) favor the synthesis of LTA structure. It is interesting to note that CHA structure can be transformed into LTA structure at 150 °C while the contrary case occurs at 190 °C. The formation mechanism of CHA phase synthesized at 150 °C seems different from that at 190 °C because the two CHA phases exhibit distinct thermal stability. For the former, its formation should be related with the layered prephase,14,15 while for the latter it can be explained by the relatively high thermal stability of CHA phase. The SEM images in Fig. 1 indicate that the samples T170-24h, T190-6h and T190-12h exhibit typical cubic shapes while T190-24h and T190-48h are perfect triclinic crystals. For the sample T150-24h, both triclinic and irregular crystals can be observed. Above XRD and SEM results indicate that high-quality pure CHA phase can be synthesized at a higher temperature. In the subsequent optimization, the crystallization temperature and time were kept at 190 °C and 24 h.
 | ||
| Fig. 1 XRD patterns and SEM images of products synthesized under different crystallization temperature and time. | ||
Seed crystals were introduced to the synthesis mixture but ionic liquids were not added any more in the following investigation, considering that morpholine is a suitable structure-directing agent of CHA-type molecular sieve and seed crystals can accelerate the crystallization process and reduce the template consumption. As shown in Fig. 2 and Table 1, the product is the mixture of CHA phase and prephase (sample Seed0-IL0) when neither ionic liquid nor seed crystals are added. As a comparison, when 0.095 g of seeds (as-synthesized T190-24h) is added into the synthesis gel, the diffraction peak of the prephase decreases obviously, and the major product becomes CHA phase. It should be noted that the ratio of morpholine/Al2O3 is as low as 1 in this synthesis, which is much less than the template amount (morpholine/Al2O3 = 2.5) reported by Jin et al.10 On the other hand, no any CHA phase can be produced when only seed crystals are introduced. SEM images in Fig. 2 indicate that the sample Seed5-IL0 contains more large triclinic-shape crystals and less amorphous-like particles as compared with the sample Seed0-IL0, confirming the beneficial effect of seed crystals.
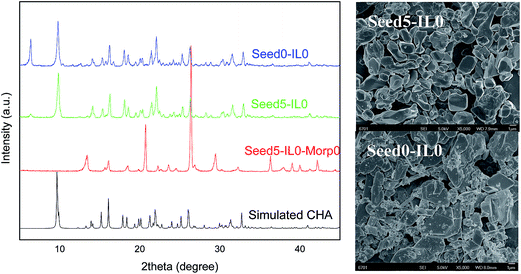 | ||
| Fig. 2 XRD patterns of products synthesized in the presence of seeds but absence of ILs together with two typical SEM images. | ||
3.2 Composition and pore texture
Among all samples listed in Table 1, four samples reveal relatively pure CHA phase. The inorganic-sources utilizations of these samples are summarized in Table 2. It can be observed from this table that they are all beyond 90%, verifying the high zeolite yield benefits from the solvent-free synthesis route. From the point view of cost savings, the synthesis conditions for the sample Seed5-IL0 is the most potential. In the following study, these samples are selected and used for further analysis.| Sample | Calcined product yields (g) | Utilization of inorganic sourcesa (%) | Langmuir surface area (m2 g−1) | Micropore volumeb (cm3 g−1) |
|---|---|---|---|---|
| a The utilization calculated from the weight ratio of calcined product yield with total inorganic sources except for water and fluorine.b t-Plot method. | ||||
| T150-24h | 1.445 | 98.7 | 806 | 0.273 |
| T190-24h | 1.379 | 94.2 | 665 | 0.248 |
| T190-48h | 1.424 | 97.3 | 769 | 0.289 |
| Seed5-IL0 | 1.377 | 94.1 | 818 | 0.288 |
The nitrogen adsorption isotherms of the calcined T150-24h, T190-24h, T190-48h and Seed5-IL0 are type I isotherms (Fig. S1†), which demonstrate that they are typical microporous materials. The Langmuir surface area and micropore volume of these samples are in the ranges of 665–818 m2 g−1 and 0.25–0.29 cm3 g−1 (Table 2), which are higher than the data reported in the literature.10,17 The high surface area and micropore volume for the calcined Seed5-ILs0 imply that it has the potential to be used in zeolite-related applications, such as adsorption and separation.18,19
Solution 13C NMR, TG-DTA experiment and CHN elemental analysis are used to determine the type and amount of organic amines occluded in the cages of the sample T190-48h. TG-DTA experiment (Fig. 3) of the sample exhibits that a 15.5% weight loss in the 375–435 °C temperature ranges accompanied by exothermic effect can be clearly observed, which is associated with the combustion decomposition of morpholine. CHN analysis indicates that the contents of C, H and N are 10.50 wt%, 2.03 wt% and 3.10 wt% in this as-synthesized sample T190-48h. Based on these data the molar ratio of C/N and the total weight of the three elements can be calculated and they are 3.95 and 15.6 wt%, respectively. Obviously, these data are in well agreement with the composition of organic amines and weight loss in TG curve. Solution 13C NMR of the sample T190-48h was carried out to confirm the type of organic species occluded in the cages of CHA structure. As shown in Fig. 3, nothing but morpholine is observed in the cages of CHA molecular sieve. The number of about one morpholine molecule per CHA cage in the as-synthesized T190-48h can be calculated from the TG results and the structural information of the CHA framework (i.e. each unit cell has two CHA cages and is composed of 12 T atoms). The EDS analysis shows that the ratio of F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P is 0.31
P is 0.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 in the sample. On the basis of all information achieved before, an expected chemical formula of as-synthesized T190-48h can be determined to be (Al6P6O24)(C4H9NO)2 (adsorbed water is not considered). This result implies that CHA molecular sieve perhaps can be synthesized in the absence of F− ions. Further optimizing the synthesis and understanding the role of seed crystals are still necessary in the future.
28 in the sample. On the basis of all information achieved before, an expected chemical formula of as-synthesized T190-48h can be determined to be (Al6P6O24)(C4H9NO)2 (adsorbed water is not considered). This result implies that CHA molecular sieve perhaps can be synthesized in the absence of F− ions. Further optimizing the synthesis and understanding the role of seed crystals are still necessary in the future.
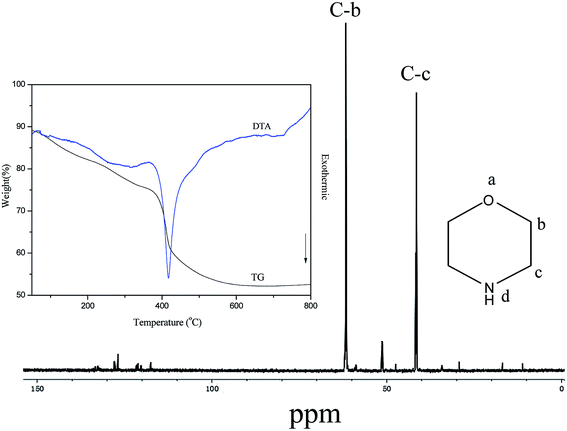 | ||
| Fig. 3 Solution 13C NMR and TG-DTA curves of as-synthesized CHA-type aluminophosphate molecular sieve (sample T190-48h). | ||
4. Conclusions
In conclusion, aluminophosphate molecular sieve with CHA topology has been synthesized with seed-assisted solvent-free route. The synthetic route reported here demonstrates that it can effectively reduce the consumption of water and organic amines, which may open up a new opportunity for the preparation of other molecular sieves in a cost-saving and environmentally benign way.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21306072, 21203081) and Development Program of Lanzhou University of Technology for excellent teachers (Grant No. Q201113).References
- S. T. Wilson, B. M. Lok, C. A. Messina, T. R. Cannan and E. M. Flanigen, J. Am. Chem. Soc., 1982, 104, 1146–1147 CrossRef CAS.
- D. Fan, P. Tian, X. Su, Y. Yuan, D. Wang, C. Wang, M. Yang, L. Wang, S. Xu and Z. Liu, J. Mater. Chem. A, 2013, 1, 14206–14213 CAS.
- L. Wu and E. J. M. Hensen, Catal. Today, 2014, 235, 160–168 CrossRef CAS.
- Z. Li, J. Martinez-Triguero, P. Concepcion, J. Yu and A. Corma, Phys. Chem. Chem. Phys., 2013, 15, 14670–14680 RSC.
- T. Alvaro-Munoz, E. Sastre and C. Marquez-Alvarez, Catal. Sci. Technol., 2014, 4, 4330–4339 CAS.
- S.-T. Yang, J.-Y. Kim, H.-J. Chae, M. Kim, S.-Y. Jeong and W.-S. Ahn, Mater. Res. Bull., 2012, 47, 3888–3892 CrossRef CAS.
- S. Lin, J. Li, R. P. Sharma, J. Yu and R. Xu, Top. Catal., 2010, 53, 1304–1310 CrossRef CAS.
- J. Wu, H. Zhao, N. Li, Q. Luo, C. He, N. Guan and S. Xiang, CrystEngComm, 2012, 14, 8671–8676 RSC.
- X. Zhao, J. Zhao, J. Wen, A. Li, G. Li and X. Wang, Micropor. Mesopor. Mater., 2015, 213, 192–196 CrossRef CAS.
- Y. Jin, Q. Sun, G. Qi, C. Yang, J. Xu, F. Chen, X. Meng, F. Deng and F. S. Xiao, Angew. Chem., 2013, 52, 9172–9175 CrossRef CAS PubMed.
- Q. Wu, X. Liu, L. Zhu, L. Ding, P. Gao, X. Wang, S. Pan, C. Bian, X. Meng, J. Xu, F. Deng, S. Maurer, U. Muller and F. S. Xiao, J. Am. Chem. Soc., 2015, 137, 1052–1055 CrossRef CAS PubMed.
- L. Ren, Q. Wu, C. Yang, L. Zhu, C. Li, P. Zhang, H. Zhang, X. Meng and F. S. Xiao, J. Am. Chem. Soc., 2012, 134, 15173–15176 CrossRef CAS PubMed.
- R. E. Morris and S. L. James, Angew. Chem., 2013, 52, 2163–2165 CrossRef CAS PubMed.
- Ø. B. Vistad, D. E. Akporiaye and K. P. Lillerud, J. Phys. Chem. B, 2001, 105, 12437–12447 CrossRef.
- Ø. B. Vistad, D. E. Akporiaye, F. Taulelle and K. P. Lillerud, Chem. Mater., 2003, 15, 1639–1649 CrossRef.
- M. Harding and B. Kariuki, Acta Crystallogr C, 1994, 50, 852–854 CrossRef.
- K. Egeblad, M. Kustova, S. K. Klitgaard, K. Zhu and C. H. Christensen, Microporous Mesoporous Mater., 2007, 101, 214–223 CrossRef CAS.
- S. R. Venna and M. A. Carreon, J. Phys. Chem. B, 2008, 112, 16261–16265 CrossRef CAS PubMed.
- L. Zhang, J. N. Primera-Pedrozo and A. J. Hernandez-Maldonado, J. Phys. Chem. C, 2010, 114, 14755–14762 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra18148h |
| This journal is © The Royal Society of Chemistry 2015 |
