Growth of MoS2@C nanobowls as a lithium-ion battery anode material†
Chunyu Cuia,
Xiu Lia,
Zhe Hub,
Jiantie Xu*b,
Huakun Liub and
Jianmin Ma*ab
aSchool of Physics and Electronics, Hunan University, Changsha, China 410082. E-mail: nanoelechem@hnu.edu.cn; Jianm@uow.edu.au
bInstitute for Superconducting and Electronic Materials, University of Wollongong, Wollongong, Australia 2500. E-mail: jx307@uowmail.edu.au
First published on 21st October 2015
Abstract
Layered MoS2 has attracted much attention as a promising anode material for lithium ion batteries. The intrinsically poor electrical/ionic conductivity, volume expansion and pulverization, stress accumulation and unstable solid–electrolyte interface formation within MoS2 electrodes during the lithiation–delithiation process significantly result in their fast capacity fading, poor rate capability and cycle life. To address these critical issues, a novel nanobowl structure for MoS2 with a carbon coating (MoS2@C-400, 500, 600) is successfully fabricated by a facile solvothermal method, followed by a post-annealing process. The fabricated MoS2@C-600 and MoS2@C-500 exhibited high reversible capacities of 1164.4 and 1076.4 mA h g−1 at 0.2C, and maintained high capacity retention of 72.1% and 78.4% over 150 cycles, respectively. Such remarkable lithium storage properties are attributed to the unique nanobowl structure, which provides a large accessible surface area and high pore volume, and flexible carbon film coating, allowing for easy diffusion of electrolyte, alleviation of volume expansion, formation of stable solid electrolyte interfaces and fast diffusion of lithium ions.
1. Introduction
Lithium-ion batteries (LIBs) have attracted increasing research interest and become one of the main power supplies for smart electronic devices and electric vehicles (EVs) and hybrid EVs (HEVs) due to their advantages of high power/energy density, long cycling life, and environmental friendliness.1 In order to acquire even higher energy density of LIBs, the development of high-energy electrode materials is the key to success.2 In the commercial market, graphite as an anode material has been commonly used in LIBs for the past two decades owing to its rich natural resources, low cost, and good cycling stability. Its low theoretical capacity (∼372 mA h g−1), poor rate performance and plating (deposition) of metallic Li at ∼0.05 V (vs. Li+/Li), however, limit its further application in high-energy EVs and HEVs. Graphene, a crystalline allotrope of carbon with 2-dimensional (2D) properties, has been widely investigated for energy conversion/storage applications due to its unique physical and chemical properties.3 In terms of its application as anode for LIBs, graphene exhibited much higher capacity and rate capability than graphite because of its larger surface area, higher electronic conductivity, and remarkable thermal and mechanical stability.4Analogue to graphene, two-dimensional transition-metal layered transition-metal dichalcogenides have also been widely investigated as anode materials in reversible storage of Li to replace the graphite, as well as graphene.5 MoS2, one of the MX2-type (M = Ti, Nb, Mo, Ta; X = S, Se, Te) compounds, possesses a layered crystal structure that is stacked layer-by-layer in the order of ‘S–Mo–S’, in which the S–S layers are supported by van der Waals forces and Mo atoms are sandwiched in the center between S–S layers.6 These structural features provide strong molecular bonds within a single layer but weak interlayer bonds, and thus facilitate the insertion/extraction of Li+ ions, leading to a high theoretical capacity of ∼670 mA h g−1 based on 4 Li+ ions reacted with every MoS2 molecule. Owing to its high theoretical capacity, as well as its rich natural resources, MoS2 has been extensively demonstrated to be an ideal anode material for LIBs.7
To date, intrinsically poor electrical/ionic conductivity, volume expansion and pulverization, stress accumulation and unstable solid–electrolyte interface formation within MoS2 electrode during the lithiation–delithiation process significantly result fast capacity fading, poor rate capability and cycle life. To solve these critical issues, many efforts have been made, including morphology control,8–13 hybridizing MoS2 nanostructures with more conductive materials (e.g., porous carbon, carbon nanotubes, and graphene),8–18 and enlarging the lattice in the c-direction between S–S layers.19 Among these efforts, nanostructure engineering and hybridization with more conductive materials of MoS2 have been proven to be an effective method. Typically, hollow structures for electrode materials have been widely demonstrated to be beneficial to enhance electrical/ionic conductivity and buffer volume expansion. The hollow structures not only provide free spaces to alleviate the structural strain during the lithium insertion/extraction process, but also facilitate the fast diffusion of lithium ions in large accessible areas and by short diffusion paths, leading to improved rate capability and cycling stability. Despite the structural advantages of hierarchical hollow MoS2,12,17 as well as MoS2 microspheres,8 MoS2/polyaniline nanowires,10 MoS2/carbon nanoflake,13,18 honeycomb-like MoS2,11 and MoS2/graphene nanoflowers, the further enhancement of the capacity, rate capability and cycling stability of MoS2 is still highly desirable.
Here, for the first time, we synthesized novel nanobowl structures for MoS2@C by a facial solvothermal method, followed by an annealing process. Apart from the structural advantages of hollow spheres or other closed hollow structures, the bowl-like nanostructure of MoS2@C with open interior and exterior surfaces provides extra excellent/enhanced characteristics to further enhance the electrochemical performance. For example, it includes: (i) higher accessible specific surface area and surface to volume ratio than the hollow structure, offering more accessible areas from the interfacial contact areas to facilitate the quick penetration of electrolyte and more lithium ion storage; (ii) amorphous carbon films coated on not only the exterior, but also the interior surfaces of MoS2 to protect electrodes from pulverization and enhance the conductivity of MoS2, enabling fast electron transportation across the interface of the MoS2 and the carbon sheets; and (iii) a ‘sandwich-like’ amorphous carbon coating to enhance the mechanical flexibility and maintain the structural integrity of MoS2, as well as alleviating its volume changes. Therefore, by integrating these advantages of both nanostructure engineering and hybridization, we found that the MoS2@C-600 exhibited the high initial capacity of 1558.3 mA h g−1 at 0.1C and 1164.4 mA h g−1 at 0.2C while MoS2@C-500 maintained a high reversible capacity of 846 mA h g−1 at 0.2C over 150 cycles, corresponding to an initial capacity retention of 78.4%. The capacity and cycle stability was considerably enhanced compared to MoS2–graphene (808 mA h g−1 retained after 100 cycles, 100 mA g−1),16 and MoS2–C (698 mA h g−1 retained after 60 cycles, 100 mA g−1).14
2. Experimental section
2.1 Synthesis of MoS2
Firstly, sodium molybdate and thioacetamide were dissolved in deionized water and N-methyl pyrrolidone (NMP) with continuous stirring for 30 minutes. Secondly, the obtained mixture was transferred into a 50 mL Teflon-lined stainless steel autoclave and maintained at 200 °C for 24 h. Thirdly, the reaction system was naturally cooled down to room temperature. Finally, the obtained products were collected by centrifugation, washed with distilled water and ethanol, and dried at 60 °C under vacuum.2.2 Synthesis of MoS2@C
Firstly, 0.1 g of the as-prepared MoS2 and 5 mL oleic acid were mixed, and the mixture was kept under stirring for 24 h in a 40° thermostatic water bath. Secondly, the obtained mixture was collected by centrifugation and washed with ethanol, which was followed by an annealing process in Ar atmosphere at a heating rate of 3° min−1 at temperatures of 400°, 500°, and 600° for 2 h, and finally, naturally cooling to room temperature.2.3 Materials characterizations
The morphologies and structures of the as-prepared samples were characterized by scanning electron microscopy (SEM) on a Hitachi S-4800, with both transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) on a JEOL2010. The phase of the as-prepared samples was identified by X-ray diffraction (XRD) using a Rigaku D max-2500. Raman spectra were collected on a Lab RAM HR 800 Raman spectrometer. X-ray photoelectron spectra (XPS) were acquired on an ESCALAB 250. The Brunauer−Emmett−Teller (BET) measurements were carried out using a Micromeritics ASAP 2020 system. Pore size distribution (PSD) curves were obtained from N2 adsorption data by the density functional theory (DFT) method (Micromeritics). Thermogravimetric analysis (TGA, Setaram018124) was performed at a heating rate of 10 °C min−1 in air.2.4 Electrochemical measurements
The electrodes were fabricated by blending the MoS2, MoS2@C-400, MoS2@C-500, or MoS2@C-600 with acetylene black carbon and polyvinylidene difluoride (PVDF), in a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. N-Methyl-2-pyrrolidone (NMP) was used as the blending solvents for the mixture. The obtained slurry was coated on Cu foil, dried at 90C for 12 h, and cut into electrodes. The obtained electrodes were dried again at 90C for 12 h in vacuum. CR 2032 coin-type cells were assembled using the as-prepared electrode as the working electrode, Li foil as the counter electrode and reference electrode, porous polypropylene film as the separator, and 1 M LiPF6 in a 1
1, respectively. N-Methyl-2-pyrrolidone (NMP) was used as the blending solvents for the mixture. The obtained slurry was coated on Cu foil, dried at 90C for 12 h, and cut into electrodes. The obtained electrodes were dried again at 90C for 12 h in vacuum. CR 2032 coin-type cells were assembled using the as-prepared electrode as the working electrode, Li foil as the counter electrode and reference electrode, porous polypropylene film as the separator, and 1 M LiPF6 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v/v) mixture of ethylene carbonate (EC), ethylene carbonate (EC), and diethyl carbonate (DEC) as the electrolyte. The performance of the cells was measured using an automatic battery tester system (Land®, China) and galvano statically charged and discharged at various current densities in the voltage range of 0.02–3.0 V, with 1C = 500 mA g−1. Cyclic voltammograms (CV) and electrochemical impedance spectra (EIS) were collected using a CHI 760C (CH Instruments, USA) electrochemical workstation over a frequency range of 50 mHz to 1 MHz. Owing to the low amount of carbon, only ∼5 wt%, the capacity contribution from carbon was neglected. Moreover, the specific capacity and C-rates of MoS2@C were calculated based on the total mass of MoS2@C.
1 (v/v/v) mixture of ethylene carbonate (EC), ethylene carbonate (EC), and diethyl carbonate (DEC) as the electrolyte. The performance of the cells was measured using an automatic battery tester system (Land®, China) and galvano statically charged and discharged at various current densities in the voltage range of 0.02–3.0 V, with 1C = 500 mA g−1. Cyclic voltammograms (CV) and electrochemical impedance spectra (EIS) were collected using a CHI 760C (CH Instruments, USA) electrochemical workstation over a frequency range of 50 mHz to 1 MHz. Owing to the low amount of carbon, only ∼5 wt%, the capacity contribution from carbon was neglected. Moreover, the specific capacity and C-rates of MoS2@C were calculated based on the total mass of MoS2@C.
3. Results and discussion
The fabrication process of MoS2@C nanobowls is simply illustrated in Fig. 1. The MoS2 nanobowls were initially synthesized by a solvothermal reaction using sodium molybdate (SM) and thioacetamide (TAA) in N-methyl pyrrolidone (NMP)/H2O at 200 °C for 24 h. To explore the influence of synthesis conditions on the morphology and structure of MoS2, a series of MoS2 prepared by using different volume ratios of solvents (NMP/H2O, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 2
3, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 3
2, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and mass ratios of solutes (SM/TAA, 1
0) and mass ratios of solutes (SM/TAA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1
1.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2
2, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 4
2, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) are compared in Experimental section (ESI, Table S1†). Based on the optimal synthesis condition, the formation mechanism of MoS2 nanobowls is proposed. Initially, the growth in the [001] or [002] direction of MoS2 is strongly hindered because of the blocking effect of –OH on the crystal planes (e.g., (001) or (002)), leading to the formation of MoS2 monolayers.20 In the following step, the adjacent MoS2 monolayers that bonded by van der Waals forces self-assemble to form nanoflakes, then the MoS2 nanoflakes stack and form the sheet structure that is thinner at the edge and thicker in the middle.21 Driven by ever-growing thermodynamic forces, the thinner edges of the MoS2 sheets become curled up to make the 2D architectures more stable, and the lamellar structures at the edge gradually grow upwards. Simultaneously, the thicker middle of the MoS2 sheets forms as the bottom of a sphere-shaped bowl. Since the surface energy of MoS2 reaches a fixed value with MoS2 ever growing, the unique nanobowl structure of the MoS2 remains stable. In order to further enhance the anode performance of MoS2, a series of the as-synthesized MoS2 nanobowls with carbon coating (MoS2@C) are prepared by annealing mixtures of MoS2/oleic acid (OA) at 400 °C, 500 °C, and 600 °C. During this annealing process, the OA is expected to be carbonized to a thin carbon film that uniformly coated inner and outer surfaces of the MoS2 nanobowls. More details of the synthesis procedure are presented in the Experimental section. The obtained MoS2@C annealed at 400 °C, 500 °C and 600 °C were denoted as MoS2@C-400, MoS2@C-500, and MoS2@C-600, respectively.
4) are compared in Experimental section (ESI, Table S1†). Based on the optimal synthesis condition, the formation mechanism of MoS2 nanobowls is proposed. Initially, the growth in the [001] or [002] direction of MoS2 is strongly hindered because of the blocking effect of –OH on the crystal planes (e.g., (001) or (002)), leading to the formation of MoS2 monolayers.20 In the following step, the adjacent MoS2 monolayers that bonded by van der Waals forces self-assemble to form nanoflakes, then the MoS2 nanoflakes stack and form the sheet structure that is thinner at the edge and thicker in the middle.21 Driven by ever-growing thermodynamic forces, the thinner edges of the MoS2 sheets become curled up to make the 2D architectures more stable, and the lamellar structures at the edge gradually grow upwards. Simultaneously, the thicker middle of the MoS2 sheets forms as the bottom of a sphere-shaped bowl. Since the surface energy of MoS2 reaches a fixed value with MoS2 ever growing, the unique nanobowl structure of the MoS2 remains stable. In order to further enhance the anode performance of MoS2, a series of the as-synthesized MoS2 nanobowls with carbon coating (MoS2@C) are prepared by annealing mixtures of MoS2/oleic acid (OA) at 400 °C, 500 °C, and 600 °C. During this annealing process, the OA is expected to be carbonized to a thin carbon film that uniformly coated inner and outer surfaces of the MoS2 nanobowls. More details of the synthesis procedure are presented in the Experimental section. The obtained MoS2@C annealed at 400 °C, 500 °C and 600 °C were denoted as MoS2@C-400, MoS2@C-500, and MoS2@C-600, respectively.
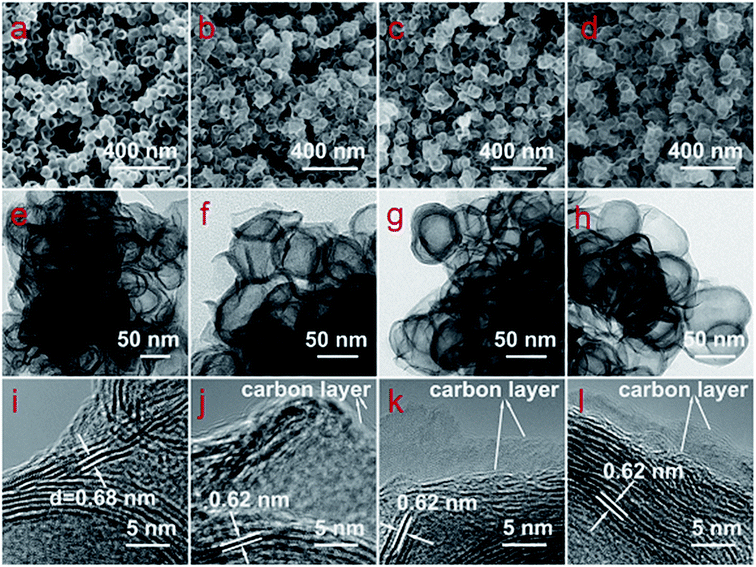
The morphologies and structures of the as-prepared MoS2 and MoS2@C samples are systematically investigated by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). As can be seen, Fig. 2a–d displays that all MoS2, MoS2@C-400, MoS2@C-500 and MoS2@C-600 are composed of the nanobowls with a uniform size of ∼50 nm in diameter. As expected, there were no distinct morphology changes for MoS2 and MoS2@C, indicating the excellent structural stability of MoS2 nanobowls after annealing. The detailed structures and sizes of MoS2 and MoS2@C nanobowls were also further confirmed by TEM (Fig. 2e–h) and high-resolution TEM (HR-TEM) (Fig. 2i–l). As shown in Fig. 2i, MoS2 was composed of a ring-like structure with 3–7 lamellar layers. The ring-like structures have a lattice spacing of 0.68 nm between lamellar layers corresponding to a distance between (002) planes of MoS2. After pyrolysis of the carbon precursor, the lattice spacing of MoS2@C was slightly decreased from 0.68 to 0.62 nm. The decreased lattice spacing could be ascribed to re-crystallization and removal of structural defects of MoS2.8,13,15,16
Fig. 3a displays the XRD patterns of MoS2, MoS2@C-400, MoS2@C-500, and MoS2@C-600. As shown in Fig. 3a, MoS2 exhibits four distinct peaks at 9.0°, 17.8°, 32.5°, and 57.5°. Two broadened peaks in the high-angle regions (32.5° and 57.0°) correspond to the (100) and (110) planes, respectively. The peak at 17.8° is indexed to the reflection of (002) plane while the peak at 9.0° is assigned to the (001) plane.14 After the annealing process, all the XRD peaks of MoS2@C-400, MoS2@C-500, and MoS2@C-600 are well indexed to hexagonal MoS2 (JCPDS card no. 73-1508), including a 14.9° main peak, which can be assigned to the (002) plane. As can be seen, the presence of the peak at 14.9° but disappearance of two peaks at 9° and 17.8° are due to the removal of structural defects and recrystallization of MoS2 after the annealing process, leading to the decrease in the layer-by-layer lattice spacing.17 These results are consistent with the HRTEM results (Fig. 2i–l). Raman spectroscopy (Fig. 3b) reveals the MoS2 structure with the appearance of two characteristic peaks at 379 cm−1 and 403 cm−1, corresponding to the E12g and A1g modes, respectively.17 As expected, MoS2@C composites all display typical E12g and A1g modes in the same region. In addition, the characteristic D band (a defect/disorder peak) at 1370 cm−1 and G band (a graphitic peak) at 1580 cm−1 further confirm the presence of carbon in the MoS2@C. The intensity ratio of ID/IG decreased from MoS2@C-400, MoS2@C-500, to MoS2@C-600. It is mainly due to higher degree of carbonization of carbon nanosheets at higher annealing temperature. As expected, the high crystallization of carbon is beneficial to improve electrical conductivity of MoS2@C.22 FT-IR spectroscopy was employed to explore the chemical functional groups of MoS2@C composites (especially, uniform carbon coating film on the surfaces), as shown in Fig. 3c. The peaks of the FT-IR spectra represent various functional groups. As expected, MoS2@C-600 displays various functional groups, including, the O–H bond at 3403 cm−1 C–H bond at 2918 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond at 1587 cm−1 and C–O bond at 1147 cm−1. Compared to MoS2@C-400 and MoS2@C-500, the more sharp peaks with high intensities for MoS2@C-600 further reveal that oleic acid are highly carbonized on the surfaces of MoS2 at higher annealing temperatures.23 In order to determine the carbon content in the MoS2@C, thermogravimetric analysis (TGA) was adopted. As shown in Fig. 3d and S3,† the carbon content of MoS2@C-400, MoS2@C-500, and MoS2@C-600 was estimated to be 15.67, 13.44, and 15.11 wt%, respectively. Apart from the confirmation by HRTEM (Fig. 2j), an additional exothermic peak in the differential thermogravimetric (DTG) curve for MoS2@C-400 (Fig. S3b†) also further confirms the lower crystallization of carbon coating for MoS2@C-400.
C bond at 1587 cm−1 and C–O bond at 1147 cm−1. Compared to MoS2@C-400 and MoS2@C-500, the more sharp peaks with high intensities for MoS2@C-600 further reveal that oleic acid are highly carbonized on the surfaces of MoS2 at higher annealing temperatures.23 In order to determine the carbon content in the MoS2@C, thermogravimetric analysis (TGA) was adopted. As shown in Fig. 3d and S3,† the carbon content of MoS2@C-400, MoS2@C-500, and MoS2@C-600 was estimated to be 15.67, 13.44, and 15.11 wt%, respectively. Apart from the confirmation by HRTEM (Fig. 2j), an additional exothermic peak in the differential thermogravimetric (DTG) curve for MoS2@C-400 (Fig. S3b†) also further confirms the lower crystallization of carbon coating for MoS2@C-400.
To investigate the chemical states of Mo, S, and C of the MoS2 and MoS2@C, high resolution X-ray photoelectron spectroscopy (XPS) measurements were carried out. As shown in Fig. 3e, the individual doublet peaks with binding energies at 162 and 163.2 eV in the S 2p spectrum are exactly assigned to the S2− ions of MoS2, whereas two main peaks with binding energies of 232.5 eV (Mo 3d3/2) and 232.5 eV (Mo 3d5/2) in the Mo 3d (Fig. 3f) spectrum are well indexed to Mo4+ of MoS2.12 As expected, the Mo and S XPS peaks Fig. S4,† and likewise, the MoS2@C-500 (Fig. S4d and e†) and MoS2@C-600 (Fig. S4g and h†) peaks are well matched with those of MoS2. In addition, the presence of carbon in the MoS2@C composites is also further confirmed by distinct XPS C1 speaks at around 285 eV in each C1s spectrum (Fig. S4c, f, and i†).
Brunauer–Emmett–Teller (BET) analysis was employed to further investigate the pore size distribution and specific surface area of MoS2 and MoS2@C. As shown in Fig. 4, a typical hysteresis loop indicating a mesoporous structure is observed in both the MoS2 sample and the MoS2@C samples. MoS2, MoS2@C-400, MoS2@C-500, and MoS2@C-600 exhibit specific surface areas of 88.21, 44.86, 49.65, and 72.75 m2 g−1, respectively. Owing to the higher degree of carbonization at higher annealing temperature, more mesopore structures can be obtained from the carbon layer, so the MoS2@C composites at higher annealing temperature feature higher BET specific surface areas and total pore volumes, as well as a wider pore size distribution (insets of Fig. 4b–d). Besides, due to the carbon coating for inner-sides of MoS2, the void volume of MoS2 is partially occupied, leading to lower surface areas for the MoS2@C than MoS2. Nevertheless, the carbon film coated on the surfaces of MoS2@C nanobowls can provide more favorable paths for electrons fast transportation, protect the electrode pulverization and enhance the mechanical properties of composites during the lithiation–delithiation process.
 | ||
| Fig. 4 BET nitrogen adsorption and desorption isotherms of (a) MoS2, (b) MoS2@C-400, (c) MoS2@C-500, and (d) MoS2@C-600. Inset of each plot: pore size distribution of the corresponding sample. | ||
Fig. S5a–d† presents typical CV curves of the MoS2, MoS2@C-400, MoS2@C-500, and MoS2@C-600 cells at 0.2 mV s−1 in the voltage range of 0.02–3.0 V, respectively. As shown in Fig. S5a,† MoS2 displays two reduction peaks at 1.1 and 0.3 V in the first cathodic process. The peak at 1.1 V can be attributed to insertion of Li ions into the interlayers of MoS2 (formation of LixMoS2), which change from trigonal prisms to octahedra in the MoS2 structure. The weak reduction peak at 1.1 V indicates the poor ionic conductivity for MoS2. The peak at 0.3 V is indicative of the conversion of LixMoS2 into metallic Mo and Li2S.10 In the following anodic process, an oxidation peak is observed at ∼1.85 V (A1) that is attributed to the oxidation of Mo to MoS2, followed by another oxidation peak at ∼2.39 V (A2) associated with the oxidation of Li2S. In the following cathodic process, the peaks at 1.1 and 0.3 V disappear, and two new peaks at about 1.9 (C1) and 1.3 V (C2) emerge, which can be ascribed to the conversion of S to Li2S and the association of Li+ ions with Mo, respectively.16 After the first anodic–cathodic cycle, highly overlapping and distinct pairs of redox peaks (A1–C2 and A2–C1) appeared in the subsequent CV curves of MoS2, indicating the good reversibility of MoS2. For the MoS2@C composites, take MoS2@C-600 (Fig. 5a or S5d†) as example, it displays three distinct reduction peaks at approximately 0.95, 0.45, and 0.05 V. Compared to MoS2, the clear peak at 0.95 V is associated with insertion of Li+ ions, while the peak at 0.45 V is indicative of the conversion process, and the formation of solid electrolyte interphase (SEI), another clear peak at 0.05 V is related to the lithium ion storage of the amorphous carbon layers. Due to the contribution of the carbon layers, extra Li+ ions can be inserted in for MoS2@C composites, so the MoS2 CV curves (Fig. S5a†) show weaker reduction peaks at 1.1 V than MoS2@C (Fig. S5b–d†). Similarly, highly overlapped CV curves in the subsequent cycles further support the high reversibility of MoS2@C. It should be noted that the oxidation peaks at ∼1.85 V in all three anodic processes and the reduction peak at 1.1 V in the second and third cathodic processes are extremely weak for all MoS2@C composites compared with that of MoS2. This is probably due to the strong interaction between lithium ions and carbon nanosheets, overlapping the oxidation peaks of Mo.
Fig. 5b presents the first discharge–charge profiles for the MoS2, MoS2@C-400, MoS2@C-500, and MoS2@C-600 at 0.1C in the voltage range of 0.02–3 V. As can be seen, the discharge–charge electrochemical behavior of MoS2 and MoS2@C were almost consistent with the cyclic voltammetry (CV) measurements. Along with the discharge–charge profiles of MoS2 and MoS2@C at 0.1C, the discharge–charge profiles of MoS2@C-600 at various C-rates from 0.2 to 10C are also presented in Fig. 5c. The electrochemical behaviors of MoS2 and MoS2@C agree well with previous reports.15,24
To compare the rate capability of MoS2 and MoS2@C, we charged and discharged the cells for 60 cycles in the voltage range of 0.02–3 V at current densities from 0.2C to 10C (Fig. 5d). As shown in Fig. 5d, the initial discharge capacities of the MoS2, MoS2@C-400, MoS2@C-500, and MoS2@C-600 are 1461.5, 1405.3, 1478.1 and 1558.3 mA h g−1 at 0.1C (assume 1C = 500 mA g−1), with initial coulombic efficiencies of 67.8%, 71.6%, 74.6%, and 75.6%, respectively. When the C rate is increased from 0.2 to 10C and changes back to 0.1C, the MoS2@C-600 exhibits the higher average charge capacities of 958.6 (0.2C), 792.5 (0.5C), 657.6 (1C), 504 (2C), 363.3 (5C), and 171.5 (10C) mA h g−1, followed by the MoS2@C-500, than MoS2@C-400 and MoS2. When the charge/discharge current density changes from 10C back to 0.1C, the specific capacity of all these four samples can be well restored, indicating extraordinarily high cycling stabilities. The detailed numerical results are listed in Table 1. To further compare their rate capabilities, the capacity retention vs. C-rates of MoS2 and MoS2@C composites is presented in Fig. S6a,† obviously MoS2@C-500 and MoS2@C-600 present higher capacity retention (as a function of C-rate) than MoS2 or MoS2@C-400. The improved rate capability is attributed to the carbon coating film, resulting the improved conductivity and enhanced mechanical properties of MoS2@C-500 or MoS2@C-600 during the discharge–charge process. Although a thin carbon film was also coated on the surface of MoS2@C-400, MoS2@C-400 presented poorer rate capability than MoS2@C-500 or MoS2@C-600 because of its lower degree of carbonization at 400 °C.
| 0.2C | 0.5C | 1C | 2C | 5C | 10C | 0.1C | |
|---|---|---|---|---|---|---|---|
| MoS2 | 850.9 | 659.9 | 537.5 | 270.1 | 99.6 | 51.0 | 910.5 |
| MoS2@C-400 | 546.7 | 344.2 | 221.1 | 140.9 | 69.4 | 67.3 | 642.6 |
| MoS2@C-500 | 892.5 | 737.1 | 679.1 | 482.6 | 353.9 | 185.3 | 988.4 |
| MoS2@C-600 | 958.6 | 792.5 | 657.6 | 504.0 | 363.3 | 171.5 | 1024.9 |
Fig. 5e presents the relatively long-term cycling performances of the MoS2 and MoS2@C at 0.2C. As can be seen, the MoS2, MoS2@C-400, MoS2@C-500, and MoS2@C-600 exhibited initial discharge capacities of 928.8, 898.3, 1076.4, and 1164.4 mA h g−1, with an initial coulombic efficiency of 69.9%, 73.5%, 76.9%, and 79.1%, respectively. After 150 cycles, the MoS2, MoS2@C-400, MoS2@C-500, and MoS2@C-600 cells can maintain discharge capacities of 621.6, 605.1, 846.0, and 839.3 mA h g−1 with initial capacity retention of 66.9%, 67.4%, 78.6%, and 72.1%, respectively. Despite the different capacities and capacity retention, MoS2 and MoS2@C all present average coulombic efficiencies of ∼99%, further indicating their highly stable structures during the lithiation–delithiation processes. Furthermore, the long-term cycling performances of MoS2, MoS2@C-400, MoS2@C-500, and MoS2@C-600 at the higher C-rate of 1C are also compared in Fig. 5e and S7.† As can be seen, MoS2@C-600 delivered a higher discharge capacity of 858.2 mA h g−1 compared with the other three samples (Fig. S7†). After 150 cycles, MoS2@C-500 maintained higher initial capacity retention of 58.5% compared with MoS2@C-600 (48.3%) over the 150 cycles, although MoS2@C-600 delivered a higher capacity than MoS2@C-500 in the initial cycles. The higher initial capacity retention at 1C indicates the excellent anode performance of MoS2@C-500 at higher C-rate. This is mainly associated with the moderate degree of crystallization of the carbon film of MoS2@C-500, which could provide good mechanical properties during the repeated discharge–charge cycles at higher C-rates, leading to the enhanced cycling stability.
To investigate the kinetics of MoS2 and MoS2@C nanobowls, electrochemical impedance spectra (EIS) were conducted on the cells before cycling, after rate capability measurements, and after long-term cycling measurements at 0.2C, over the frequency range from 1 MHz to 0.05 Hz. As shown in Fig. 6a–c, all the spectra include a depressed semicircle in the high- and medium-frequency regions, which could be assigned to the resistance associated with lithium-ion diffusion through the solid electrolyte interphase (SEI) film (Rs) and the charge transfer resistance (Rct), respectively, and a clear ∼45° inclined line in the low-frequency region, which could be considered to be Warburg impedance (W).25 The Rct is a key indicator for the kinetics of the electrode material. The Rct was calculated by using the equivalent circuit model shown in the inset of Fig. 6a. The fabricated model also includes electrolyte resistance (Re), a constant phase element (CPE-1), and a non-ideal constant phase element (CPE-2). Compared with the fresh cells before cycling (Fig. 6a), all the corresponding cells after rate measurements (Fig. 6b) or long-term cycling measurements (Fig. 6c) at 0.2C apparently show decreased Rct of the electrode materials. This is mainly due to the electrode–electrolyte activation after deep discharge–charge cycles. As estimated from the fabricated model (inset of Fig. 6a), the relatively low Rct (less than 100 Ω) of the electrodes after rate capability measurements and long-term cycling measurements further indicate the excellent structural and chemical stability of the electrode materials, facilitating ions fast transportation. The Rct values of MoS2 and MoS2@C before and after cycling increase in the order of MoS2@C-600 < MoS2@C-500 < MoS2@C-400 < MoS2 (before rate measurements) and MoS2@C-500 < MoS2@C-600 < MoS2@C-400 < MoS2 (after rate measurements), indicating highest kinetics of MoS2@C-600 or MoS2@C-600 among the series electrodes. These results are almost consistent with their electrochemical performance.
 | ||
| Fig. 6 Electrochemical impedance spectra (EIS) of MoS2, MoS2@C-400, MoS2@C-500, and MoS2@C-600: (a) before cycling, (b) after rate capability measurements (shown in Fig. 3d) and (c) after long-term cycling measurements (shown in Fig. 3e) at 0.2C. Inset of (a): equivalent circuit model. | ||
4. Conclusion
In summary, we have successfully fabricated the MoS2 and MoS2@C composites with high uniformity and a well-defined nanobowl structure through a facial solvothermal method, followed by an annealing process. Compared to MoS2, the MoS2@C presented the excellent electrochemical performance for LIBs. The MoS2@C-500 maintained a high reversible capacity of 846 mA h g−1 at 0.2C over 150 cycles, corresponding to an initial capacity retention of 78.4%. The excellent electrochemical performance of MoS2@C-500 nanobowls is attributed to their unique nanobowl structure. The nanobowl structure provides large accessible surface areas and pore volumes for easy electrolyte penetration and rapid Li+ diffusion, as well as the accommodation of volume variation during the repeated charge–discharge cycles, thus leading to enhanced capacity and rate capability. The MoS2@C nanobowls are expected to be an ideal anode material for high-energy LIBs in future.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51302079). We also thank Dr Tania Silver from Institute for Superconducting and Electronic Materials (University of Wollongong) for revising our manuscript.References
- (a) V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243 RSC; (b) M. M. Thackeray, C. Wolverton and E. D. Isaacs, Energy Environ. Sci., 2012, 5, 7854 RSC.
- (a) A. Manthiram, J. Phys. Chem. Lett., 2011, 2, 176 CrossRef CAS; (b) J. W. Fergus, J. Power Sources, 2010, 195, 939 CrossRef CAS; (c) J. Xu, S. Dou, H. Liu and L. Dai, Nano Energy, 2013, 2, 439 CrossRef CAS.
- K. S. Novoselov, V. I. Falko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, Nature, 2012, 490, 192 CrossRef CAS PubMed.
- (a) N. A. Kaskhedikar and J. Maier, Adv. Mater., 2009, 21, 2664 CrossRef CAS; (b) E. Yoo, J. Kim, E. Hosono, H.-S. Zhou, T. Kudo and I. Honma, Nano Lett., 2008, 8, 2277 CrossRef CAS PubMed; (c) Z.-S. Wu, W. Ren, L. Xu, F. Li and H.-M. Cheng, ACS Nano, 2011, 5, 5463 CrossRef CAS PubMed; (d) Z.-L. Wang, D. Xu, H.-G. Wang, Z. Wu and X.-B. Zhang, ACS Nano, 2013, 7, 2422 CrossRef CAS PubMed; (e) J. Xu, I. Y. Jeon, J. M. Seo, S. Dou, L. Dai and J. B. Baek, Adv. Mater., 2014, 26, 7317 CrossRef CAS PubMed.
- (a) R. Dominko, D. Arčon, A. Mrzel, A. Zorko, P. Cevc, P. Venturini, M. Gaberscek, M. Remskar and D. Mihailovic, Adv. Mater., 2002, 14, 1531 CrossRef CAS; (b) C. Feng, J. Ma, H. Li, R. Zeng, Z. Guo and H. Liu, Mater. Res. Bull., 2009, 44, 1811 CrossRef CAS; (c) J. Xiao, D. Choi, L. Cosimbescu, P. Koech, J. Liu and J. P. Lemmon, Chem. Mater., 2010, 22, 4522 CrossRef CAS.
- (a) A. C. Neto, Phys. Rev. Lett., 2001, 86, 4382 CrossRef; (b) Y. Liu, H. Nan, X. Wu, W. Pan, W. Wang, J. Bai, W. Zhao, L. Sun, X. Wang and Z. Ni, ACS Nano, 2013, 7, 4202 CrossRef CAS PubMed.
- (a) Y. Liu, X. Zhu, Z. Duan and X. Xie, Chem. Commun., 2013, 49, 10305 RSC; (b) X. Zhu, K. Wang, D. Yan, S.-R. Le, R.-J. Ma, K.-N. Sun and Y.-T. Liu, Chem. Commun., 2015, 51, 11888 RSC; (c) L. Pan, X.-D. Zhu, X.-M. Xie and Y.-T. Liu, J. Mater. Chem. A, 2015, 3, 2726 RSC; (d) Z.-Q. Duan, Y.-C. Sun, Y.-T. Liu, X.-M. Xie and X.-D. Zhu, RSC Adv., 2014, 4, 41543 RSC; (e) L. Pan, Y.-T. Liu, X.-M. Xie and X.-D. Zhu, Chem.–Asian J., 2014, 9, 1519 CrossRef CAS PubMed.
- S. Ding, D. Zhang, J. S. Chen and X. W. D. Lou, Nanoscale, 2012, 4, 95 RSC.
- (a) X. Fang, X. Yu, S. Liao, Y. Shi, Y.-S. Hu, Z. Wang, G. D. Stucky and L. Chen, Microporous Mesoporous Mater., 2012, 151, 418 CrossRef CAS; (b) G. Huang, T. Chen, W. Chen, Z. Wang, K. Chang, L. Ma, F. Huang, D. Chen and J. Y. Lee, Small, 2013, 9, 3693 CrossRef CAS PubMed; (c) Y. Shi, Y. Wang, J. I. Wong, A. Y. S. Tan, C.-L. Hsu, L.-J. Li, Y.-C. Lu and H. Y. Yang, Sci. Rep., 2013, 3, 2169 Search PubMed; (d) P. Wang, H. Sun, Y. Ji, W. Li and X. Wang, Adv. Mater., 2014, 26, 964 CrossRef CAS PubMed.
- L. Yang, S. Wang, J. Mao, J. Deng, Q. Gao, Y. Tang and O. G. Schmidt, Adv. Mater., 2013, 25, 1180 CrossRef CAS PubMed.
- J. Wang, J. Liu, D. Chao, J. Yan, J. Lin and Z. X. Shen, Adv. Mater., 2014, 26, 7162 CrossRef CAS PubMed.
- Z. Wan, J. Shao, J. Yun, H. Zheng, T. Gao, M. Shen, Q. Qu and H. Zheng, Small, 2014, 10, 4975 CrossRef CAS PubMed.
- C. Zhao, J. Kong, X. Yao, X. Tang, Y. Dong, S. L. Phua and X. Lu, ACS Appl. Mater. Interfaces, 2014, 6, 6392 CAS.
- (a) J. Xiao, X. Wang, X. Q. Yang, S. Xun, G. Liu, P. K. Koech, J. Liu and J. P. Lemmon, Adv. Funct. Mater., 2011, 21, 2840 CrossRef CAS; (b) K. Chang and W. Chen, Chem. Commun., 2011, 47, 4252 RSC; (c) K. Chang and W. Chen, J. Mater. Chem., 2011, 21, 17175 RSC; (d) S. Ding, J. S. Chen and X. W. D. Lou, Chem.–Eur. J., 2011, 17, 13142 CrossRef CAS PubMed; (e) D. Kong, H. He, Q. Song, B. Wang, W. Lv, Q.-H. Yang and L. Zhi, Energy Environ. Sci., 2014, 7, 3320 RSC; (f) C. Wang, W. Wan, Y. Huang, J. Chen, H. H. Zhou and X. X. Zhang, Nanoscale, 2014, 6, 5351 CAS; (g) F. Zhou, S. Xin, H. W. Liang, L. T. Song and S. H. Yu, Angew. Chem., Int. Ed., 2014, 53, 11552 CrossRef CAS PubMed.
- K. Chang, D. Geng, X. Li, J. Yang, Y. Tang, M. Cai, R. Li and X. Sun, Adv. Energy Mater., 2013, 3, 839 CrossRef CAS.
- Z. Wang, T. Chen, W. Chen, K. Chang, L. Ma, G. Huang, D. Chen and J. Y. Lee, J. Mater. Chem. A, 2013, 1, 2202 CAS.
- J. Xie, J. Zhang, S. Li, F. Grote, X. Zhang, H. Zhang, R. Wang, Y. Lei, B. Pan and Y. Xie, J. Am. Chem. Soc., 2013, 135, 17881 CrossRef CAS PubMed.
- H. Yu, C. Ma, B. Ge, Y. Chen, Z. Xu, C. Zhu, C. Li, Q. Ouyang, P. Gao and J. Li, Chem.–Eur. J., 2013, 19, 5818 CrossRef CAS PubMed.
- (a) H. Liu, D. Su, R. Zhou, B. Sun, G. Wang and S. Z. Qiao, Adv. Energy Mater., 2012, 2, 970 CrossRef CAS; (b) Z. Hu, L. Wang, K. Zhang, J. Wang, F. Cheng, Z. Tao and J. Chen, Angew. Chem., Int. Ed., 2014, 126, 13008 CrossRef.
- J. Zhang, Y. Wang, Z. Lin and F. Huang, Cryst. Growth Des., 2010, 10, 4285 CAS.
- S. K. Das, R. Mallavajula, N. Jayaprakash and L. A. Archer, J. Mater. Chem., 2012, 22, 12988 RSC.
- A. C. Ferrari and D. M. Basko, Nat. Nano, 2013, 8, 235 CrossRef CAS PubMed.
- J. Zhang, H. Yang, G. Shen, P. Cheng, J. Zhang and S. Guo, Chem. Commun., 2010, 46, 1112 RSC.
- (a) X. Xiong, W. Luo, X. Hu, C. Chen, L. Qie, D. Hou and Y. Huang, Sci. Rep., 2015, 5, 9254 CrossRef CAS PubMed; (b) J. Zhou, J. Qin, X. Zhang, C. Shi, E. Liu, N. Zhao and C. He, ACS Nano, 2015, 9, 3837 CrossRef CAS PubMed.
- J. Xu, S.-L. Chou, Q.-F. Gu, H.-K. Liu and S.-X. Dou, J. Power Sources, 2013, 225, 172 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17992k |
| This journal is © The Royal Society of Chemistry 2015 |