DOI:
10.1039/C5RA17914A
(Communication)
RSC Adv., 2015,
5, 85319-85322
Nitric oxide emission during the reductive heterogeneous photocatalysis of aqueous nitrate with TiO2†
Received
3rd September 2015
, Accepted 2nd October 2015
First published on 2nd October 2015
Abstract
For the first time, nitric oxide (NO), a precursor of nitrogen dioxide (NO2, a NIOSH-listed atmospheric pollutant), has been found to be one of the final products of the photocatalytic reduction of nitrate in water using TiO2 and formic acid as a hole scavenger.
Introduction
Heterogeneous photocatalysis (HP) constitutes a potential alternative for removal of nitrate from water or brines produced by remediation technologies.1 Most scientific studies concerning the use of HP for water treatment monitor exclusively the aqueous phase and are focused either on the transformation of a toxic metal or metalloid to a harmless species or, in the case of organic compounds, on their complete mineralization yielding water, simple inorganic acids and CO2. However, in many cases, HP can generate other gas phase products that might be harmful to humans and to the environment; the photocatalytic arsenic reduction over TiO2 generating arsine is an example.2 Therefore, the determination of the composition of the gas phase is crucial, not only considering potential health impacts and environmental implications, but also to contribute to a better understanding of the mechanism of removal of pollutants from the aqueous phase.
Nitrate is an essential nutrient for protein synthesis in plants and it is formed and retained by well-known bacteria mediated processes in the environment: nitrogen fixation and nitrification.3 However, it is used as fertilizer in intensive farming, leading to a sustained increase in its surface and groundwater concentration and to an acceleration of the nitrogen cycle resulting in aquatic and terrestrial eutrophication and global acidification.4 In relation with human health, nitrate ion is not considered harmful, but intestinal tract bacteria can easily convert it into NO2−, potentially affecting O2 transport by oxidation of Fe2+ in hemoglobin.5 For that reason, the WHO established a guideline value for drinking water of 50 mg L−1.5
Removal of nitrate by HP has led to an abundant literature that pointed at NH3 (or NH4+) and N2 as final products. TiO2 (Degussa or Evonik P25) presents values for the flatband potential of the conduction band (CB) and valence band (VB) as −0.3 V and +2.9 V (at pH 0), respectively. After UV irradiation of the semiconductor, conduction band electrons (eCB−) and valence band holes (hVB+) are created; eCB− can reduce electron acceptors (A) of suitable redox potential, while hVB+ can oxidize electron donors (D) or water, generating hydroxyl radicals (HO˙):
| | |
TiO2 + hν → TiO2 (eCB− + hVB+)
| (1) |
| | |
hVB+ + H2O → HO˙ + H+
| (3) |
If monoelectronic pathways are proposed in HP reactions, direct nitrate photocatalytic reduction by eCB− would not be possible because the redox potential for nitrate to the nitrate radical anion ( )6,7 hinders eCB− attack. Actually, poor reactivity for nitrate reduction or no reaction at all has been observed using bare TiO2 in pure water (see e.g. ref. 8 and 9 and references therein). Therefore, an electron donor or hole scavenger like an alcohol or a carboxylic acid (RH), able to generate reducing radicals (R˙) after reaction with hVB+ or HO˙, has to be added to allow indirect reduction through the following reactions:
)6,7 hinders eCB− attack. Actually, poor reactivity for nitrate reduction or no reaction at all has been observed using bare TiO2 in pure water (see e.g. ref. 8 and 9 and references therein). Therefore, an electron donor or hole scavenger like an alcohol or a carboxylic acid (RH), able to generate reducing radicals (R˙) after reaction with hVB+ or HO˙, has to be added to allow indirect reduction through the following reactions:
| | |
RH + hVB+/HO˙ → R˙ + H+/H2O
| (5) |
| | |
R˙ + NO3− → Rox + NO32−
| (6) |
where R
ox is the final organic oxidation product in solution.
Formic acid has been used as hole scavenger in previous studies8,10–13 leading to significantly higher nitrate conversions compared with other reagents. In addition, noble metal modified TiO2 has been reported to promote a high efficient reaction and an acceptable selectivity towards N2 formation.8,10,11,14 Several authors10,14 indicated different selectivities to N2 formation using bare TiO2 and using electron donors but, in fact, none of them have actually measured N2 in those conditions, and the gas phase has been incompletely characterized. To the best of our knowledge, only two groups have reported the quantification of N2 emissions from nitrate treatment with HP: Zhang et al.11 reported 99% selectivity to N2 when removing NO3− with Ag-modified TiO2, while Kominami et al.15 measured N2 emissions when using different metal-loaded TiO2, yielding 98% of N2 selectivity, calculated based on consumption of photogenerated electrons.
In this work, we demonstrate for the first time the emission of harmful concentrations of NO during the photocatalytic reduction of nitrate in water using TiO2 and formic acid as hole scavenger.
Results and discussion
Intermediates and final products of the photocatalytic removal of nitrate in the presence of formic acid
The concentration profiles for nitrate, formic acid and the intermediate (NO2−) and final products (NH4+, NO) as a function of time during the reductive TiO2-HP of nitrate were obtained for experiments starting with [NO3−]0 = 0.08 mM in the presence of [HCOOH]0 = 0.1 mM (at pH 2.8). As shown in Fig. 1(a), a simultaneous decrease of the NO3− concentration with complete degradation of HCOOH takes place during the reaction.
 |
| | Fig. 1 Time profiles of (a) [NO3−], [NH4+] and [HCOOH] in the aqueous phase during the photocatalytic reaction of nitrate over P25 and (b) NO evolution in the gas phase. Initial conditions: [NO3−]0 = 0.08 mM and [HCOOH]0 = 0.1 mM, pH 2.8 and T = 25 °C. Inset: time profile of [NO2−]. | |
The role of NO2− as aqueous reaction intermediate can be observed in Fig. 1(a) (inset), and its consumption occurs in parallel with the increase of the NH4+ concentration, the final and stable product in solution at the end of the reaction, as reported previously.1,11,13,14,16 On the other hand, NO (Fig. 1(b)) is emitted from the system and its total produced amount (NOT) was calculated using eqn (7):
| |
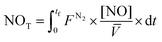 | (7) |
where NO
T is the amount of moles of NO emitted in the system,
FN2 is the N
2 flow rate (600 mL min
−1), [NO] is the temporal concentration of NO in ppmv given by the NO
x analyzer, and
![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif)
is the molar volume of NO.
At the end of the experiment (60 min), 26% of the nitrate remains in solution and the rest of the mass of nitrogen initially present as nitrate is distributed as follows: 30% of NH4+ in solution (Fig. 1), 30% of NO in the gas phase (calculated with eqn (7), representing the area below the curve in Fig. 1(b)), and the remaining 14% is supposed to be N2 or a mixture of N2 and N2O, as reported previously17 and suggested elsewhere.1,10,13,16
In the absence of light, HCOOH or TiO2, neither NO3− degradation nor formation of products was observed, indicating that NO is a byproduct of the heterogeneous photocatalytic transformation of NO3−. The similarity between the trends for the variation of [NO3−] and [HCOOH] indicates that the presence of HCOOH dictates the evolution of the other species in the system; HCOOH is the limiting reagent because when it is completely depleted the evolution of the rest of the compounds barely varies. The role of HCOOH becomes evident according to the generation of NO: the reaction initiates very fast but progressively decelerates as HCOOH is consumed, leading to a broad peak with a maximum at around 5 min of reaction (Fig. 1).
As stated previously, the monoelectronic reduction of nitrate by eCB− is not thermodynamically possible. However, formic acid reacts with hVB+18 (eqn (8)), decreasing the electron–hole recombination rate and generating CO2˙−, a very strong reducing radical 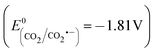 .18
.18
| | |
HCOO− + hBV+ → H+ + CO2˙−
| (8) |
CO2˙− can then reduce NO3− to NO32− (eqn (9)), this latest species being able to react rapidly in water producing NO2 (eqn (10))7 or, in the presence of O2, being reoxidized to NO3−, affecting the overall efficiency of the removal process.19 NO2 is unstable in water and can either disproportionate (eqn (11)),19–21 or react with eCB− forming NO2− (eqn (12)).22 NO2− can be reduced by eCB− (eqn (13)) generating NO22−, and this species can be hydrolyzed generating NO (eqn (14)),22 which can be also formed by NO2− thermal (eqn (15))22 or photochemical decomposition (eqn (16)).23
| | |
CO2˙− + NO3− → ˙NO32− + CO2
| (9) |
| | |
˙NO32− + H2O → ˙NO2 + 2OH−
| (10) |
| | |
2˙NO2 + H2O → NO3− + NO2− + 2H+
| (11) |
| | |
NO2− + eCB− → ˙NO22−
| (13) |
| | |
˙NO22− + H2O → ˙NO + 2OH−
| (14) |
| | |
3NO2− + 2H+ → NO3− + 2˙NO + H2O
| (15) |
| | |
NO2− + H+ + hν (365 nm) → ˙NO + HO˙
| (16) |
After these reactions, NH4+ can be formed by the one-step sequential reduction of NO by eCB−, with formation of hydroxylamine as intermediate.22 Other authors have also found that the increase on the HCOOH/NO3− molar ratio in HP experiments of nitrate removal1,10 and a working pH ≤ 3,5,13 enhances the selectivity towards NH4+.
Experiments with different initial concentrations of NO3− and HCOOH were performed in order to understand their influence on the distribution of products. The same general behavior observed in Fig. 1 was obtained for the evolution of all species (Fig. S2 and S3†). Under the studied conditions, the emission of NO was correlated with the formic acid initial concentration (Fig. 2), observing only a minor change in NOT when increasing [NO3−]0 (inset, Fig. 2), leading to 230 ± 33 μmol of NOT emitted per mmol of HCOOH.
 |
| | Fig. 2 Total NO emitted (NOT) after NO3− treatment by TiO2-HP ([TiO2] = 1 g L−1) in the presence of HCOOH. Experimental conditions: [NO3−]0 = 8 mM and [HCOOH]0 = 0.1 and 1 mM. Inset: NOT obtained at different initial NO3− concentrations and [HCOOH]0 = 0.1 mM. | |
Most of the studies of nitrate removal by TiO2-HP consider the use of formic acid for making feasible the reaction due to its high electron donor efficiency and, usually, high [HCOOH]0/[NO3−]0 ratios are used. As recently proven by Doudrik et al.,13 higher formic acid concentration does not always lead to a better nitrate removal efficiency. In addition to that observation, Fig. 2 highlights that an increase in the amount of formic acid also increases NO emissions under the studied conditions. This last consideration needs to be taken into account in the case of the possible scale up of TiO2-HP for nitrate removal in water treatment plants, due to the fact that NO is a precursor of NO2 formation in the atmosphere,24 with a NIOSH occupational exposure level of 1 ppm (recommended exposure level over a period of 15 min).25
Conclusions
The discovery of NO emissions during the removal of nitrate by TiO2-HP brings valuable information to be considered in future studies associated with the treatment of nitrate-polluted water. New TiO2 based materials, the type of electron donor and the working pH may impact on the amount of NO introduced in the gas phase or even lead to the emission of other more dangerous nitrogen oxides as NO2 or HONO. Additionally, NO appears as a major product during the removal of nitrate by HP and, therefore, its inclusion in the kinetic description of the system should be also taken into account in further works.
Acknowledgements
This work was performed as part of Agencia Nacional de Promoción Científica y Tecnológica PICT-0463 project. We thank Eng. Héctor Bajano for his guidance on NOx measurements. The first preliminary experiments were carried out at Lawrence Berkeley National Laboratory (LBNL), which operates under U.S. Department of Energy Contract DE-AC02-05CH11231. Thanks also to Marion Russel, Toshifumi Hotchi and Mohamad Sleiman for their helpful suggestions.
References
- T. Yang, K. Doudrick and P. Westerhoff, Water Res., 2013, 47, 1299 CrossRef CAS PubMed.
- K. I. Levy, M. Mizrahi, G. Ruano, G. Zampieri, F. G. Requejo and M. I. Litter, Environ. Sci. Technol., 2012, 46, 2299 CrossRef PubMed.
- A. Bernhard, The Nitrogen Cycle: Processes, Players, and Human Impact. Nat. Educ. Knowl., 2010, vol. 3, p. 25, http://www.nature.com/scitable/knowledge/library/the-nitrogen-cycle-processes-players-and-human-15644632, (accessed on 08/26/2015) Search PubMed.
- N. Gruber and J. N. Galloway, Nature, 2008, 451, 293 CrossRef CAS PubMed.
- WHO, in WHO Guidelines for Drinking-water Quality, 2011, pp. 398–403 Search PubMed.
- R. W. Fessenden and D. Meisel, J. Am. Chem. Soc., 2000, 122, 3773 CrossRef CAS.
- A. R. Cook, N. Dimitrijevic, B. W. Dreyfus, D. Meisel, L. A. Curtiss and D. Camaioni, J. Phys. Chem. A, 2001, 105, 3658 CrossRef CAS.
- M. Shand and J. A. Anderson, Catal. Sci. Technol., 2013, 3, 879 Search PubMed.
- M. I. Litter, N. Quici, J. M. Meichtry and A. M. Senn, in Photocatalysis: Fundamentals and Perspectives, ed. J. Schneider, D. Bahnemann, J. Ye, G. Li Puma and D. Dionysiou, Royal Society, chapter: Photocatalytic removal of metallic and other inorganic pollutants, accepted Search PubMed.
- J. Sá, C. A. Agüera, S. Gross and J. A. Anderson, Appl. Catal., B, 2009, 85, 192 CrossRef.
- F. Zhang, R. Jin, J. Chen, C. Shao, W. Gao, L. Li and N. Guan, J. Catal., 2005, 232, 424 CrossRef CAS.
- F. Zhang, Y. Pi, J. Cui, Y. Yang, X. Zhang and N. Guan, J. Phys. Chem. C, 2007, 111, 3756 CrossRef CAS.
- K. Doudrick, T. Yang, K. Hristovski and P. Westerhoff, Appl. Catal., B, 2013, 136–137, 40 CrossRef CAS.
- K. Doudrick, O. Monzón, A. Mangonon, K. Hristovski and P. Westerhoff, J. Environ. Eng., 2012, 138, 852 CrossRef CAS.
- H. Kominami, T. Nakaseko, Y. Shimada, A. Furusho, H. Inoue, S.-Y. Murakami, Y. Kera and B. Ohtani, Chem. Commun., 2005, 3, 2933 RSC.
- N. Wehbe, M. Jaafar, C. Guillard, J.-M. Herrmann, S. Miachon, E. Puzenat and N. Guilhaume, Appl. Catal., A, 2009, 368, 1 CrossRef CAS.
- H. Kominami, H. Gekko and K. Hashimoto, Phys. Chem. Chem. Phys., 2010, 12, 15423 RSC.
- L. L. Perissinotti, M. Brusa and M. A. Grela, Langmuir, 2001, 17, 8422 CrossRef CAS.
- M. C. Gonzalez and A. M. Braun, J. Photochem. Photobiol., A, 1996, 93, 7 CrossRef CAS.
- M. C. Gonzalez and A. M. Braun, J. Photochem. Photobiol., A, 1996, 95, 67 CrossRef CAS.
- H. H. Mohamed, C. B. Mendive, R. Dillert and D. W. Bahnemann, J. Phys. Chem. A, 2011, 115, 2139 CrossRef CAS PubMed.
- S. Goldstein, D. Behar, T. Rajh and J. Rabani, J. Phys. Chem. A, 2015, 119, 2760 CrossRef CAS PubMed.
- G. Rubasinghege and V. H. Grassian, J. Phys. Chem. A, 2009, 113, 7818 CrossRef CAS PubMed.
- A. P. Altshuller, J. Air Pollut. Control Assoc., 1956, 6, 97 CrossRef CAS.
- National Institute for Occupational Safety and Health http://www.cdc.gov/niosh/npg/npgd0454.html, (accessed August 2015).
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, Fig. S1–S3. See DOI: 10.1039/c5ra17914a |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here.  )6,7 hinders eCB− attack. Actually, poor reactivity for nitrate reduction or no reaction at all has been observed using bare TiO2 in pure water (see e.g. ref. 8 and 9 and references therein). Therefore, an electron donor or hole scavenger like an alcohol or a carboxylic acid (RH), able to generate reducing radicals (R˙) after reaction with hVB+ or HO˙, has to be added to allow indirect reduction through the following reactions:
)6,7 hinders eCB− attack. Actually, poor reactivity for nitrate reduction or no reaction at all has been observed using bare TiO2 in pure water (see e.g. ref. 8 and 9 and references therein). Therefore, an electron donor or hole scavenger like an alcohol or a carboxylic acid (RH), able to generate reducing radicals (R˙) after reaction with hVB+ or HO˙, has to be added to allow indirect reduction through the following reactions: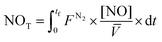
![[V with combining macron]](https://www.rsc.org/images/entities/i_char_0056_0304.gif) is the molar volume of NO.
is the molar volume of NO.
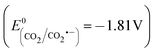 .18
.18

