Synthesis of novel isophorone-based dyes for dye-sensitized solar cells†
Yih-Chun
Chen
,
Yan-Zuo
Lin
,
Yu-Ting
Cheng
and
Yuan Jay
Chang
*
Department of Chemistry, Tung Hai University, Taichung 407, Taiwan. E-mail: jaychang@thu.edu.tw; Fax: +886-4-23590426; Tel: +886-4-23590121 ext. 32224
First published on 30th October 2015
Abstract
Four organic dyes containing isophorone as the π-bridge unit were synthesised and their photophysical and electrochemical properties were characterised. They were then used to fabricate dye-sensitised solar cells. Arylamine derivatives and cyanoacrylic acid functioned as electron donors (D) and electron acceptors (A), respectively, to form a D–π–A system without the need for a Pd catalyst. YC-1, with a long chain hexyloxy group and a strong phenothiazine donor moiety, improved both the open-circuit voltage (Voc) and short-circuit current (Jsc) and reduced charge recombination. The optimum device had a Jsc of 14.86 mA cm−2, a Voc of 0.67 V, a fill factor of 0.62 and a photon-to-current conversion efficiency of 60% at 385–605 nm, corresponding to an overall conversion efficiency of 6.18%. The photophysical properties of the dye-sensitised solar cells were analysed using a time-dependent density functional theory model with the B3LYP functional. The electronic nature of the devices was elucidated using electrochemical impedance spectroscopy and controlled intensity modulated photo spectroscopy.
1. Introduction
The global problems of the limited supply of fossil fuels and the need to reduce CO2 emissions have prompted the investigation and development of renewable and environmentally friendly sources of energy. Solar energy is widely acknowledged as a potential alternative energy resource. Compared with silicon-based photovoltaic devices, dye-sensitized solar cells (DSSCs) have received considerable attention because they are easy to fabricate, have a wide absorption range because their structures can be tuned, are colourful and transparent, and can be manufactured at low cost.1 O'Regan and Grätzel2 reported that DSSCs based on ruthenium-based complexes, such as N3, N719, black dye and Z907, yielded a high power conversion efficiency of >11%. Diau et al.3 used a porphyrin dye and achieved a maximum performance of 13%. However, another paper on a metal–complex sensitizer reported a complicated synthetic route, difficult purification and a low yield.2dThe photosensitizer is the most crucial factor determining the performance of DSSCs and numerous metal-free organic dyes have been investigated and shown to have a high performance, similar to that of N719.4 Metal-free organic dyes are environmentally friendly, low cost, have a high structural flexibility and can be easily prepared. Most organic dyes have linear-shaped molecules consisting of a strong dipole, an electron donor (D), a π-bridge, an electron acceptor (A) and a D–π–A system. To achieve an optimum D–π–A system for DSSCs, it is critical to improve the short-circuit current (Jsc) and open-current voltage (Voc).
In general, the π-bridge unit in the sensitizer has mainly been based on the use of thiophene,5 furan,6 pyrrole,7 benzene,8 diketopyrrolopyrrole9 or benzothiadiazole10 as linkers. However, the C–C bond that connects the bridges unit between the donor or acceptor moiety of sensitizers is usually formed by Pd-catalysed cascade reactions, such as Sonogashira, Heck, Suzuki–Miyaura or Stille coupling reactions. Although a Pd-based cross-coupling reaction is a powerful method for linking two moieties through a simple, time-efficient and versatile synthesis route, Pd is expensive and not environmentally friendly.
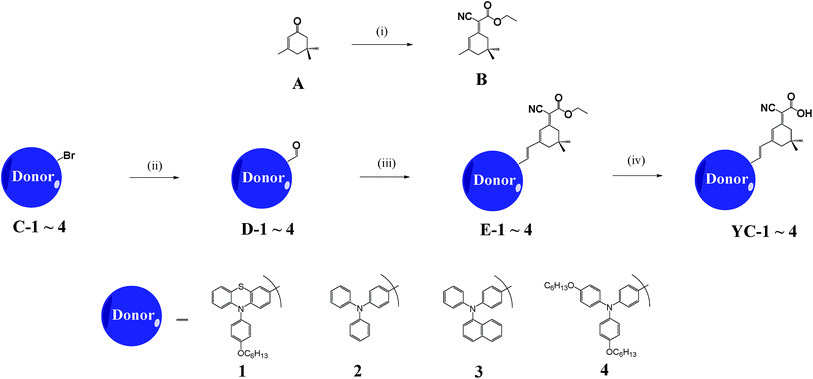
Data on the use of the simple and low-cost isophorone structure as the π-bridge for DSSCs are rare. Tian and co-workers11,12 reported high efficiencies for NKX-2753 and D-3, which incorporated coumarin and indoline derivatives, respectively, as the donor groups. In this study, we modified and designed four YC series dyes using isophorone as the π-bridge between the arylaimine electron donor and the cyanoacrylic acid acceptor without using a Pd catalyst. We have previously reported the use of phenothiazine, which has electron-rich nitrogen and sulphur heteroatoms and a molecular structure with a slight bend in the central ring.13 A long chain increases the solubility of a structure and reduces the dark current by covering the TiO2 surface.4h,14 We therefore investigated whether the introduction of an arylamine (YC-4) or phenothiazine (YC-1) with long alkoxy chains into DSSCs enhanced their light-harvesting function and Voc; YC-2 and YC-3 served as references. The synthetic procedures are described in Scheme 1 and all the structures were confirmed based on spectroscopic data.
2. Experimental
Characterisation and reagents
All reactions and manipulations were conducted under a nitrogen atmosphere and all solvents were freshly distilled according to standard procedures. 1H- and 13C-NMR spectra were recorded on Bruker spectrometers (AV 300/AV 400/AVIII HD 400/AV 500 MHz) using CDCl3 as the solvent. Chemical shifts (δ) were reported downfield from the peak with respect to tetramethylsilane. The absorption spectra were recorded on a Shimadzu UV-1800 spectrophotometer and the emission spectra and photoluminescence quantum yield were obtained using a Hitachi F-4500 spectrofluorometer. The redox potentials were measured by cyclic voltammetry on a CHI 620 analyser. All measurements were conducted in tetrahydrofuran solutions containing 0.1 M tetrabutylammonium hexafluorophosphate as the supporting electrolyte under ambient conditions after purging for 10 min with N2. A conventional three-electrode configuration was used; this consisted of a glassy carbon working electrode, a platinum counter electrode and an Ag/Ag+ reference electrode calibrated using ferrocene/ferrocenium as an internal reference. Mass spectra were recorded using a JEOL JMS-700 double-focusing mass spectrometer.Ethyl-2-cyanoacetate, ammonium acetate, benzene, n-butyllithium (1.6 M in hexane), N,N-dimethylformamide, piperidine, lithium hydroxide (LiOH), acetic anhydride and acetonitrile (MeCN) were separately purchased from ACROS, Alfa, Merck, Lancaster, TCI, Sigma-Aldrich and Showa. Chromatographic separations were performed using pre-coated silica gel plates (Kieselgel si 60, 40–63 µm; Merck).
Fabrication and characterisation of DSSCs
Fluorine-doped tin oxide (FTO) conducting glass (TCO22-7; sheet resistance, 7 Ω square−1) and Ti-nanoxide T/SP and Ti-nanoxide R/SP titanium oxide pastes were purchased from Solaronix S.A. (Switzerland). A thin film of TiO2 (10–12 µm transparent + 5 µm scattering) was coated on a 0.25 cm2 FTO glass substrate, which was then immersed in a THF or CH2Cl2 solution containing 3 × 10−4 M dye sensitisers for 12 h, then rinsed with anhydrous acetonitrile and dried. Another piece of FTO glass with a 100 nm thick layer of sputtered Pt was used as a counter electrode. The active area was controlled at dimensions of 0.25 cm2 by adhering a 60 µm thick piece of polyester tape to the Pt electrode. The photocathode was placed on top of the counter electrode and tightly clipped to form a cell; an electrolyte was then injected into the seam between the two electrodes. An acetonitrile solution containing LiI (0.5 M), I2 (0.05 M) and 4-tert-butylpyridine (TBP; 0.5 M) was used as electrolyte 1 (E1). A solution of 3-dimethylimidazolium iodide (1.0 M), LiI (0.05 M), I2 (0.03 M), guanidinium thiocyanate (0.1 M) and TBP (0.5 M) in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) valeronitrile (85
valeronitrile (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, v/v) was used as electrolyte 2 (E2). Devices composed of the commercial dye N719 under the same conditions (3 × 10−4 M; Solaronix) were used as references. The cell parameters were determined under incident light with an intensity of 100 mW cm−2 (measured using a thermopile probe; Oriel 71964) generated by a 300 W solar simulator (Oriel Sol3A Class AAA Solar Simulator 9043A, Newport) and passed through an AM 1.5 filter (Oriel 74110). The light intensity was further calibrated using an Oriel reference solar cell (Oriel 91150) and adjusted to 1.0 Sun. The monochromatic quantum efficiency was recorded using a monochromator (Oriel 74100) under short-circuit conditions. The electrochemical impedance spectra of the DSSCs were recorded using an impedance/charge extraction method (CEM)/intensity-modulated photovoltage spectroscopy (IVMS) analyser (Zahner Ennium).
15, v/v) was used as electrolyte 2 (E2). Devices composed of the commercial dye N719 under the same conditions (3 × 10−4 M; Solaronix) were used as references. The cell parameters were determined under incident light with an intensity of 100 mW cm−2 (measured using a thermopile probe; Oriel 71964) generated by a 300 W solar simulator (Oriel Sol3A Class AAA Solar Simulator 9043A, Newport) and passed through an AM 1.5 filter (Oriel 74110). The light intensity was further calibrated using an Oriel reference solar cell (Oriel 91150) and adjusted to 1.0 Sun. The monochromatic quantum efficiency was recorded using a monochromator (Oriel 74100) under short-circuit conditions. The electrochemical impedance spectra of the DSSCs were recorded using an impedance/charge extraction method (CEM)/intensity-modulated photovoltage spectroscopy (IVMS) analyser (Zahner Ennium).
Quantum chemistry computations
The structures of the dyes were optimized using the B3LYP/6-31G* hybrid functional. For the excited states, time-dependent density functional theory with the B3LYP functional was used. All analyses were performed using Q-Chem 3.0 software. The frontier orbital plots of the highest (HOMO) and lowest (LUMO) occupied molecular orbitals were drawn using GaussView 04.3. Results and discussion
Synthesis
The structures of the dyes are shown in Fig. 1 and their synthetic sequences are outlined in Scheme 1. The YC series contained an isophorone moiety as the bridge unit in the sensitizer. Its synthesis started with isophorone yielding an ester unit B (E/Z 1/1) through Knoevenagel condensation and the isomer ratio was verified using proton NMR (Fig. S1†).12 A bromo group was replaced with an aldehyde on the donor moiety of D-1–4 by n-butyl lithium and DMF. The donor part was then connected to the π-bridge and acceptor part by using the Knoevenagel condensation reaction to yield E-1–4. Four final products were obtained at high yield and were easily purified through the base hydrolysis of an ester to construct the cyanoacrylic acid acceptor unit. The structures of all new compounds were characterised spectroscopically.Absorption spectra
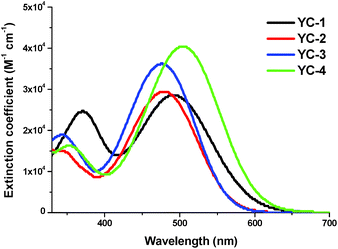
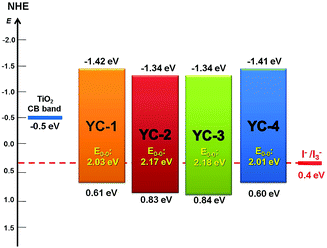
Fig. 2 shows the ultraviolet-visible absorption spectra of all dyes in CH2Cl2 solution and Fig. 3 shows the ultraviolet-visible absorption spectra of all dyes on TiO2; the photochemical and electrochemical parameters are listed in Table 1. All the sensitizers exhibited broad, high-intensity absorption peaks in the range 344–504 nm. The short wavelength region at 344–370 nm was attributed to localized π–π* transitions, whereas the long wavelength region in the range 476–504 nm was attributed to intramolecular charge-transfer transitions (ICTs). Compared with the other three compounds, the intensity of the π–π* transition of YC-1 was stronger with the phenothiazine donor moiety, indicating that the phenothiazine donor increased π-overlapping relative to the fan structure of triphenylamine. YC-1 and YC-4 showed broader and higher intensity ICT transitions because the alkoxy chain improved not only the donating ability of the donor moiety, but also the coplanar conformation of the bridge moiety between the donor and acceptor. A slightly different optimized structure was evidenced by theoretical computations using time-dependent density functional theory with the B3LYP functional in combination with the standard 6-31G* basis set (see Fig. 5). The phenomenon was consistent with the dipole moments revealed by the computation results (Table S1†). The absorption photocurrent (Jsc) and the intensity of absorption showed the same trends in solution.15| Dye | λ max a (nm)/ε (M−1 cm−1) | λ max (TiO2) | E ox b (V) | E 0–0 (eV) | E red c (V) | J sc (mA cm−2) E1/E2e | V oc (V) E1/E2 | FF E1/E2 | η d (%) E1/E2 | Loading amount (10−7 mol cm−2) |
|---|---|---|---|---|---|---|---|---|---|---|
a Absorption and emission are in CH2Cl2.
b Oxidation potential in THF (10−3 M) containing 0.1 M (n-C4H9)4NPF6 at a scan rate of 50 mV s−1 (vs. NHE).
c
E
red calculated by Eox − E0–0.
d Performance of DSSCs measured in a 0.25 cm2 working area on an FTO (7 Ω square−1) substrate.
e Electrolyte 1 (E1): LiI (0.5 M), I2 (0.05 M) and TBP (0.5 M) in MeCN. Electrolyte 2 (E2): 3-dimethylimidazolium iodide (1.0 M), LiI (0.05 M), I2 (0.03 M), guanidinium thiocyanate (0.1 M) and TBP (0.5 M) in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) valeronitrile (85 valeronitrile (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, v/v).
f See ref. 20. 15, v/v).
f See ref. 20.
|
||||||||||
| YC-1 | 491 (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) 500) |
436 | 0.61 | 2.03 | −1.42 | 14.86/11.91 | 0.67/0.69 | 0.62/0.63 | 6.18/5.17 | 13.4 |
| YC-2 | 478 (29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) 300) |
416 | 0.83 | 2.17 | −1.34 | 12.18/10.15 | 0.64/0.71 | 0.63/0.64 | 4.92/4.61 | 6.3 |
| YC-3 | 476 (36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) 300) |
405 | 0.84 | 2.18 | −1.34 | 12.43/10.78 | 0.65/0.67 | 0.63/0.61 | 5.08/4.43 | 8.3 |
| YC-4 | 504 (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) 300) |
434 | 0.60 | 2.01 | −1.41 | 13.44/12.42 | 0.66/0.69 | 0.60/0.61 | 5.37/5.20 | 11.8 |
| N719 | — | — | 1.10f | 2.60f | −1.50f | 16.22/16.32 | 0.73/0.75 | 0.59/0.60 | 7.02/7.34 | — |
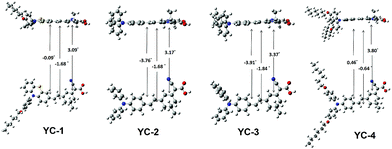 | ||
| Fig. 5 Optimized structure of YC series organic dyes estimated by time-dependent DFT/B3LYP (6-31G* basis set). | ||
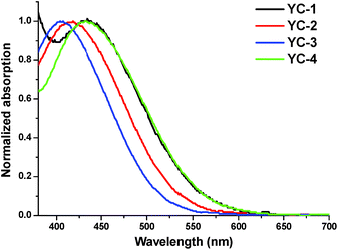
The absorption spectra of all the dyes absorbed on the TiO2 surface are shown in Fig. 3. Compared with the absorption spectra of YC-2 and YC-3, those of YC-1 and YC-4 are broader, representing advantageous spectral properties for light harvesting. All the dyes exhibited a blue shift of approximately 55–71 nm in the film ICT absorption band with respect to those in solution and the spectral differences between the dyes decreased. The blue shift, a common phenomenon for most organic dyes, appears to be a result of either the deprotonation of the carboxylic acid or strong interactions between the dye and the semiconductor surface when it was anchored onto the TiO2 surface.
Electrochemical properties
The oxidation potentials (Eox), corresponding to the HOMO level of the dyes, were measured using cyclic voltammetry. It is clear that the long hexyloxy chain in the arylamine donor moiety effectively influences the electron delocalization and oxidation potential. The lowest ionization potentials in CH2Cl2 solutions decreased in the order of YC-2 ≈ YC-3 > YC-4 ≈ YC-1 (Fig. 4 and S19†). The LUMO levels of the dyes were estimated according to the Eox values and the zero–zero band gaps at the start of the absorption spectra (Table 1). YC-1 and YC-4 had a narrower HOMO–LUMO energy gap, consistent with the wider absorption ranges in Fig. 2. This observation was confirmed by theoretical calculations. For appropriate functioning of the DSSCs, all the sensitizers were maintained at a more positive potential than the redox potential of the iodine/iodide electrolyte (0.4 V vs. NHE) to ensure a sufficient driving force to regenerate the oxidized dyes from the electrolytes. The LUMO levels of all sensitizers were higher than the conduction bands (−0.5 V vs. NHE) of TiO2. The energy levels of all dyes were in accordance with the requirements for efficient electron flow.Computational analysis
The effects of YC-1–4 on the device performance were further explored through theoretical models. Complete geometrical optimizations were performed using the B3LYP/6-31G* hybrid functional implanted in Q-Chem 3.0.16 The optimized molecular geometry of YC-1–4 is shown in Fig. 5, which suggests that the conformation of the isophorone bridge unit is nearly coplanar. However, the orientation of the dimethyl group on the slightly bulky isophorone ring prevents intramolecular aggregation. The electron distributions of frontier orbitals are shown in Fig. S17† and, as depicted, the electron densities in the HOMOs were mainly distributed around the amine donor moieties and those in the LUMOs were mainly distributed around the cyanoacrylic acid of the acceptor moieties. A delicate balance between charge separation and recombination must be maintained by modifying the molecular structures. A disturbance in the π-conjugation, either by a distortion of the molecular geometry or an interruption of the intramolecular aggregation by a long chain, may reduce the charge migration rate. As long as the charge-separated state has a longer lifetime, a higher device quantum efficiency can be achieved.17 A difference in the Mulliken charge distribution between the ground state (S0) and excited state (S1) is plotted in Fig. 6 based on the estimate generated using the time-dependent DFT/B3LYP model (see Table S2†). YC-1 and YC-4 showed the optimum charge separation in the ICT state (bar chart, Fig. 6). Both compounds had a slightly higher Voc value than YC-2 and YC-3 in DSSCs using E1.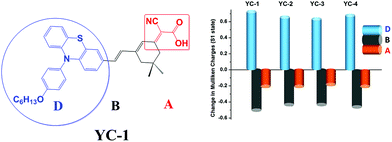 | ||
| Fig. 6 Difference of Mulliken charges between ground state (S0) and excited state (S1) estimated by the time-dependent DFT/B3LYP model. | ||
Photoexcitation pumps an electron from the HOMO to the LUMO and therefore shifts a considerable number of electrons from the donor to the acceptor. Consequently, the HOMO–LUMO energy gap in YC-1 and YC-4 was lower than that in YC-2 and YC-3 and the ICT band in the former structures exhibited a bathochromic shift with respect to the latter. This also explains the dipole moment (YC-1, 12.8343 D; YC-4, 11.9272 D) and zero–zero energy of the YC series sensitizers.
The probability of transition by excitation was estimated using the time-dependent DFT/B3LYP model and the calculated oscillator strength (f) is given in Table S1.† The f values for the lowest energy transitions of the ICT transitions (the S1 state) are all relatively high, ranging from 0.61 to 1.13. The results are consistent with the absorptivity measured in solution.
Photovoltaic performance of DSSCs
DSSC devices composed of the synthesised dyes were fabricated on the surface of TiO2 according to a standard procedure.Two types of electrolytes were used to investigate the performance of the devices: E1 consisted of LiI (0.5 M), I2 (0.05 M) and TBP (0.5 M) in MeCN, whereas E2 was composed of 3-dimethylimidazolium iodide (1.0 M) and guanidinium thiocyanate (0.1 M) in addition to LiI (0.05 M), I2 (0.03 M) and TBP (0.5 M) in a mixed solvent of MeCN and valeronitrile (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, v/v). Experiments involving co-absorption with deoxycholic acid (DCA) were conducted. The incident photocurrent conversion efficiencies (IPCEs) and photocurrent–voltage (J–V) curves of all the dyes were measured under AM 1.5 solar light (100 mW cm−2); the short-circuit current (Jsc), open-circuit voltage (Voc), fill factor (FF) and solar-to-electrical photocurrent density (η) are summarised in Table 1, where N719 serves as the reference dye.
15, v/v). Experiments involving co-absorption with deoxycholic acid (DCA) were conducted. The incident photocurrent conversion efficiencies (IPCEs) and photocurrent–voltage (J–V) curves of all the dyes were measured under AM 1.5 solar light (100 mW cm−2); the short-circuit current (Jsc), open-circuit voltage (Voc), fill factor (FF) and solar-to-electrical photocurrent density (η) are summarised in Table 1, where N719 serves as the reference dye.
The J–V plots and IPCEs of all devices with E1 are shown in Fig. 7. An apparent improvement in the Voc values was observed using E2; the values were approximately 0.02–0.05 V higher than those obtained using E1 (Table 1). The different interactions in different solvents might cause this variation in the chemical and physical properties between the sensitizer and the TiO2 surface.18 Two types of solvents were used: THF and CH2Cl2. The devices fabricated using CH2Cl2 gave the optimum results (Table S3†). Therefore the parameters of all the YC series dyes listed in Tables 1 and 2 are those of devices using the CH2Cl2 solvent system.
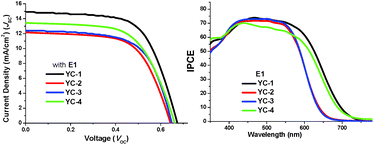 | ||
| Fig. 7 J–V plots and IPCE of DSSCs devices of YC-1–4 with the E1 electrolyte. The plots were measured under a light intensity of 1.0 Sun. | ||
| Dye | DCAa (mM) | J sc (mA cm−2) | V oc (V) | FF | η b (%) |
|---|---|---|---|---|---|
| a Concentration of dye 3 × 10−4 M in CH2Cl2. b Performance of DSSCs measured in a 0.25 cm2 working area on an FTO (7 Ω square−1) substrate under AM 1.5 irradiation with E1. | |||||
| YC-1 | 0 | 14.86 | 0.67 | 0.62 | 6.18 |
| 10 | 12.91 | 0.67 | 0.63 | 5.40 | |
| YC-2 | 0 | 12.18 | 0.64 | 0.63 | 4.92 |
| 10 | 12.83 | 0.66 | 0.64 | 5.36 | |
| YC-3 | 0 | 12.43 | 0.65 | 0.63 | 5.08 |
| 10 | 12.56 | 0.66 | 0.63 | 5.28 | |
| YC-4 | 0 | 13.44 | 0.66 | 0.60 | 5.37 |
| 10 | 13.17 | 0.65 | 0.60 | 5.17 | |
An apparent improvement in the Voc values was observed using E2; specifically, the values were approximately 0.02–0.07 V higher those obtained using E1. The lower concentration of Li ions in E2 raised the Fermi energy level of the conduction band of TiO2, enhancing the Voc values and subsequently increasing the gap between the conduction band of TiO2 and E2.19 Although the Voc values of all devices with E2 were considerably higher than those with E1, the Jsc values were considerably lower, particularly that of YC-2 (approximately 17%) (see Table 1).
The long hexyloxy chains of YC-1 and YC-4 may prevent contact between I3− and the surface of TiO2; these compounds exhibited higher Voc values than those of the other two sensitisers, which had no long chain.4c,4j,21 Therefore YC-1 and YC-4 reduce the dark current by lowering the rate of charge recombination by more than YC-2 and YC-3.
The alignment of dyes on the surface of TiO2 is a crucial factor in the further optimisation of the performance of DSSCs. The sensitiser must be carefully placed vertically on TiO2 to prevent intermolecular aggregation under dark current conditions. It was examined whether adding a DCA co-absorbent prevented the dye from lying on the TiO2 surface. For some dyes that are unable to form high-quality films, adding DCA has been reported to effectively improve the film morphology and consequently reduce the charge recombination rate.22 However, adding DCA may not improve the performance of devices that have a high-quality film morphology. Tian and co-workers12,23 reported the same structure using the YC-2 dye; however, they did not report the effects of adding a DCA co-absorbent on S1 or D-2. In this study, we showed that adding DCA (10 mM) to YC-2 (up to 5.36%) and YC-3 (5.28%) improved the quantum efficiency by 4–9% (Table 2 and Fig. 8). In the presence of DCA, both the Voc and Jsc values increased considerably, indicating an improvement in film quality and dye loading (Table S4†). This indicates that the addition of a DCA co-absorbent helps the dye to align vertically on the TiO2 surface rather than lying horizontally. YC-1 and YC-4 seem to form a high-quality monolayer. On adding DCA, both the Voc and FF values remained nearly the same; however, the Jsc values decreased from 14.86 to 12.91 mA cm−2 for YC-1 and from 13.44 to 13.17 mA cm−2 for YC-4. The quantum efficiency showed an overall decrease of 4–13%. The reduction in the Jsc value can be explained by a lower amount of loading because the TiO2 surface was partly occupied by DCA. The long hexyloxy substituents in YC-1 and YC-4 not only cover the TiO2 surface, but also form a high-quality film. This effect is supported by the higher resistance in the electrochemical impedance spectra. The relative photovoltaic efficiency of YC-1 with E1 was higher than that with the other electrolytes; this difference was attributed to the higher efficiency of electron injection to the conduction band of TiO2. YC-1 exhibited an IPCE of 60% in the region 385–605 nm, a Jsc value of 14.86 mA cm−2, a Voc value of 0.67 V and an FF of 0.62, corresponding to an overall conversion efficiency of 6.18%.
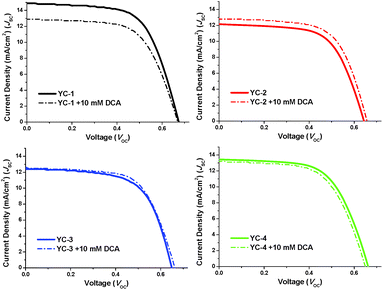 | ||
| Fig. 8 J–V curves of DSSCs fabricated with and without a DCA co-absorbent at a light intensity of 1.0 Sun. | ||
Electrochemical impedance spectroscopy and lifetime using CEM/IMVS
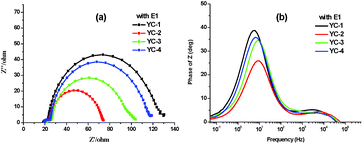
Electrochemical impedance spectroscopy was performed to further elucidate the photovoltaic properties of the dyes (Fig. 9). The Nyquist and Bode plots were measured under forward bias and dark conditions. In the Nyquist plots, a semicircle was observed for each dye; this semicircle was associated with the transport process at the TiO2/electrolyte/dye interface; a larger semicircle corresponds to a larger charge recombination resistance at a low frequency, indicating a lower charge recombination rate and smaller dark current.24 The radius of the larger semicircles increased in the order YC-2 < YC-3 < YC-4 < YC-1 and this trend was consistent with the Voc values in E1. The electron lifetime can be directly estimated by fitting the plots to the equation τ = 1/(2πf) in a Bode plot (Fig. 9b), where a shift to a lower frequency corresponds to a longer electron lifetime. The larger Voc values of YC-1 (27.5 ms) and YC-4 (22.4 ms) correspond to a longer electron lifetime and the results showed that the long hexyloxy chain of the arylamine donor could prevent direct contact between iodine and the TiO2 surface and reduce the charge recombination rate.We measured the electron lifetime (τr) by IMVS (Fig. 10a). The electron lifetime decreased in the order YC-1 (31.41 ms) > YC-4 (24.62 ms) > YC-3 (18.66 ms) > YC-2 (16.77 ms) under the same light intensity; a similar trend was seen in the data by fitting Bode plots. The Voc value is defined as the energy difference between the Fermi level of TiO2 and the redox couple of the electrolyte.18 Considering that the redox couple in the fabricated cells was identical (E1 electrolyte), the difference in Voc for YC-1–4 should be attributable to the electron density in TiO2. We also performed CEM measurements to investigate the influence of the Voc value on the shift in the Femi energy level of the TiO2 conduction band. The conduction band edge of TiO2 exhibited a higher up-shift for YC-1 (Fig. 10b) and this trend was consistent with the Nyquist plots. The high Voc value and electron lifetime were associated with the balance between electron injection and charge recombination.
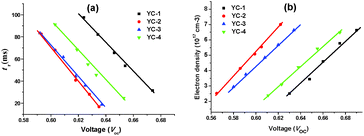 | ||
| Fig. 10 IMVS and CEM results for the YC series dyes using E1 in CH2Cl2 without DCA. (a) Electron lifetime as a function of Voc measured by IMVS. (b) Electron density as a function of Voc by CEM. | ||
4. Conclusions
We have shown that high-performance DSSCs can be achieved using isophorone as the bridge moiety in YC-1–4 dyes and that the dyes can be synthesised without a Pd catalyst. The planar shape of isophorone, with two slightly bulky methyl substituents, resulted in the formation of films on the TiO2 surface. Both YC-1 and YC-4 exhibited remarkable solar-to-energy conversion efficiencies as a result of their favourable light-harvesting capacity and high absorptivity. The YC-1 and YC-4 sensitisers formed high-quality films on TiO2 without the use of a DCA co-absorbent. A typical device fabricated using YC-1 had an IPCE of 60% in the region 385–605 nm, a Jsc of 14.86 mA cm−2, a Voc of 0.67 V and an FF of 0.62, corresponding to an overall conversion efficiency of 6.18%. YC-1 also showed higher Voc values and electron lifetimes using the impedance/CEM/IMVS method. Although YC-1 showed the highest performance of 6.18%, this was still lower than NKX-2753 and D-3. However, we used low-cost C–C bond coupling without the requirement for a Pd catalyst to obtain the isophorone derivatives. This facilitated the preparation of useful dyes for solar cells and our investigation of the donor side with long alkyl chain led to simplified device fabrication without the addition of DCA.Acknowledgements
Financial support from the Ministry of Science and Technology of Taiwan (MOST 103-2113-M-029-007-MY2) and Academia Sinica are gratefully acknowledged. Special thanks to Professor C.-P. Hsu at the Institute of Chemistry Sinica of Taiwan for her assistance with the quantum chemistry computations.References
- M. Grätzel, Inorg. Chem., 2005, 44, 6841 CrossRef PubMed.
- (a) B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef; (b) M. K. Nazeeruddin, A. Key, I. Rodicio, R. Humphry-Baker, E. Müller, P. Liska, N. Vlachopoulous and M. Grätzel, J. Am. Chem. Soc., 1993, 115, 6382 CrossRef CAS; (c) M. K. Nazeeruddin, S. M. Zakeeruddin, R. Humphry-Baker, M. Jirousek, P. Liska, N. Vlachopoulous, V. Shklover, C. H. Fischer and M. Grätzel, Inorg. Chem., 1999, 38, 6298 CrossRef CAS PubMed; (d) M. K. Nazeeruddin, P. Péchy, T. Renouard, S. M. Zakeeruddin, R. Humphry-Baker, P. Comte, P. Liska, L. Cevey, E. Costa, V. Shklover, L. Spiccia, G. B. Deacon, C. A. Bignozzi and M. Grätzel, J. Am. Chem. Soc., 2001, 123, 1613 CrossRef CAS PubMed; (e) P. Wang, S. M. Zakeeruddin, J. E. Moser, M. K. Nazeeruddin, T. Sekiguchi and M. Grätzel, Nat. Mater., 2003, 2, 402 CrossRef CAS PubMed.
- A. Yella, H. W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W. G. Diau, C. Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629 CrossRef CAS PubMed.
- (a) Z. S. Wang, Y. Cui, K. Hara, Y. Dan-oh, C. Kasada and A. Shinpo, Adv. Mater., 2007, 19, 1138 CrossRef CAS; (b) D. Kuang, S. Uchida, R. Humphry-Baker, S. M. Zakeeruddin and M. Grätzel, Angew. Chem., Int. Ed., 2008, 47, 1923 CrossRef CAS PubMed; (c) H. Choi, C. Baik, S. O. Kang, J. Ko, M.-S. Kang, M. K. Nazeeruddin and M. Grätzel, Angew. Chem., Int. Ed., 2008, 47, 327 CrossRef CAS PubMed; (d) S. Ito, H. Miura, S. Uchida, M. Takata, K. Sumioka, P. Liska, P. Comte, P. Péchy and M. Grätzel, Chem. Commun., 2008, 5194 RSC; (e) H. Qin, S. Wenger, M. Xu, F. Gao, X. Jing, P. Wang, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2008, 130, 9202 CrossRef CAS PubMed; (f) Y. J. Chang and T. J. Chow, Tetrahedron, 2009, 65, 4726 CrossRef CAS; (g) H. Tian, X. Yang, J. Cong, R. Chen, J. Liu, Y. Hao, A. Hagfeldt and L. Sun, Chem. Commun., 2009, 6288 RSC; (h) Y. Wu, M. Marszalek, S. M. Zakeeruddin, Q. Zhang, H. Tian, M. Grätzel and W. Zhu, Energy Environ. Sci., 2012, 5, 8261 RSC; (i) N. Cai, R. Li, Y. Wang, M. Zhang and P. Wang, Energy Environ. Sci., 2013, 6, 139 RSC; (j) Y. J. Chang, P.-T. Chou, Y.-Z. Lin, M. Watanabe, C.-J. Yang, T.-M. Chin and T. J. Chow, J. Mater. Chem., 2012, 22, 21704 RSC; (k) X.-H. Zhang, Z.-S. Wang, Y. Cui, N. Koumura, A. Furube and K. Hara, J. Phys. Chem. C, 2009, 113, 13409 CrossRef CAS; (l) R. Y.-Y. Kin, H.-W. Lin, Y.-S. Yen, C.-H. Chang, H.-H. Chou, P.-W. Chen, C.-Y. Hsu, Y.-C. Chen, J. T. Lin and K.-C. Ho, Energy Environ. Sci., 2013, 6, 2477 RSC; (m) S. Cai, X. Hu, Z. Zhang, J. Su, X. Li, A. Islam, L. Han and H. Tian, J. Mater. Chem. A, 2013, 1, 4763 RSC.
- (a) N. Koumura, Z.-S. Wang, S. Mori, M. Miyashita, E. Suzuki and K. Hara, J. Am. Chem. Soc., 2006, 128, 14256 CrossRef CAS PubMed; (b) Z.-S. Wang, N. Koumura, Y. Cui, M. Takahashi, H. Sekiguchi, A. Mori, T. Kubo, A. Furube and K. Hara, Chem. Mater., 2008, 20, 3993 CrossRef CAS.
- (a) J. T. Lin, P.-C. Chen, Y.-S. Yen, C.-Y. Hsu, H.-H. Chou and M.-C. P. Yeh, Org. Lett., 2009, 11, 97 CrossRef CAS PubMed; (b) S. Qu, C. Qin, A. Islam, Y. Wu, W. Zhu, J. Hua, H. Tian and L. Han, Chem. Commun., 2012, 48, 6972 RSC.
- (a) H. Li, Y. Hou, Y. Yang, R. Tang, J. Chen, H. Wang, H. Han, T. Peng, Q. Li and Z. Li, ACS Appl. Mater. Interfaces, 2013, 5, 12469 CrossRef CAS PubMed; (b) H. Li, L. Yang, R. Tang, Y. Hou, Y. Yang, H. Wang, H. Han, J. Qin, Q. Li and Z. Li, Dyes Pigm., 2013, 99, 863 CrossRef CAS.
- Y. J. Chang, Y. J. Wu, P.-T. Chou, M. Watanabe and T. J. Chow, Thin Solid Films, 2014, 558, 330 CrossRef CAS.
- (a) S. Qu, W. Wu, J. Hua, C. Kong, Y. Long and H. Tian, J. Phys. Chem. C, 2010, 114, 1343 CrossRef CAS; (b) S. Qu, B. Wang, F. Guo, J. Li, W. Wu, C. Kong, Y. Long and J. Hua, Dyes Pigm., 2012, 92, 1384 CrossRef CAS; (c) T. W. Holcombe, J.-H. Yum, Y. Kim, K. Rakstys and M. Grätzel, J. Mater. Chem. A, 2013, 1, 13978 RSC.
- (a) M. Velusamy, K. R. Justin Thomas, J. T. Lin, Y.-C. Hsu and K.-C. Ho, Org. Lett., 2005, 7, 1899 CrossRef CAS PubMed; (b) W. Zhu, Y. Wu, S. Wang, W. Li, X. Li, J. Chen, Z.-S. Wang and H. Tian, Adv. Funct. Mater., 2011, 21, 756 CrossRef CAS; (c) S. Haid, M. Marszalek, A. Mishra, M. Wielopolski, J. Teuscher, J.-E. Moser, R. Humphry-Baker, S. M. Zakeeruddin, M. Grätzel and P. Bäuerle, Adv. Funct. Mater., 2012, 22, 1291 CrossRef CAS; (d) S. Qu, W. Wu, J. Hua, C. Kong, Y. Long and H. Tian, J. Phys. Chem. C, 2010, 114, 1343 CrossRef CAS.
- Z.-S. Wang, K. Hara, Y. Dan-oh, C. Kasada, A. Shinpo, S. Suga, H. Arakawa and H. Sugihara, J. Phys. Chem. B, 2005, 109, 3907 CrossRef CAS PubMed.
- B. Liu, W. Zhu, Q. Zhang, W. Wu, M. Xu, Z. Ning, Y. Xie and H. Tian, Chem. Commun., 2009, 1766 RSC.
- C.-J. Yang, Y. J. Chang, M. Watanabe, Y.-S. Hon and T. J. Chow, J. Mater. Chem., 2012, 22, 4040 RSC.
- (a) M. Akhtaruzzaman, Y. Seya, N. Asao, A. Islam, E. Kwon, A. El-Shafei, L. Han and Y. Yamamoto, J. Mater. Chem., 2012, 22, 10771 RSC; (b) W. Li, Y. Wu, X. Li, Y. Xie and W. Zhu, Energy Environ. Sci., 2011, 4, 1830 RSC.
- (a) T. Kitamura, M. Ikeda, K. Shigaki, T. Inoue, N. A. Anderson, X. Ai, T. Lian and S. Yanagida, Chem. Mater., 2004, 16, 1806 CrossRef CAS; (b) S. L. Li, K. J. Jiang, K. F. Shao and L. M. Yang, Chem. Commun., 2006, 2792 RSC; (c) S. Kim, H. Choi, D. Kim, K. Song, S. O. Kang and J. Ko, Tetrahedron, 2007, 63, 9206 CrossRef CAS; (d) K. Hara, Z. S. Wang, T. Sato, A. Furube, R. Katoh, H. Sugihara, Y. Dan-oh, C. Kasada, A. Shinpo and S. Suga, J. Phys. Chem. B, 2005, 109, 15476 CrossRef CAS PubMed; (e) D. P. Hagberg, T. Edvinsson, T. Marinado, G. Boschloo, A. Hagfeldt and L. Sun, Chem. Commun., 2006, 2245 RSC.
- Y. Shao, L. F. Molnar, Y. Jung, J. Kussmann, C. Ochsenfeld, S. T. Brown, A. T. B. Gilbert, L. V. Slipchenko, S. V. Levchenko, D. P. ONeill, R. A. DiStasio Jr, R. C. Lochan, T. Wang, G. J. O. Beran, N. A. Besley, J. M. Herbert, C. Y. Lin, T. V. Voorhis, S. H. Chien, A. Sodt, R. P. Steele, V. A. Rassolov, P. E. Maslen, P. P. Korambath, R. D. Adamson, B. Austin, J. Baker, E. F. C. Byrd, H. Dachsel, R. J. Doerksen, A. Dreuw, B. D. Dunietz, A. D. Dutoi, T. R. Furlani, S. R. Gwaltney, A. Heyden, S. Hirata, C.-P. Hsu, G. Kedziora, R. Z. Khalliulin, P. Klunzinger, A. M. Lee, M. S. Lee, W. Z. Liang, I. Lotan, N. Nair, B. Peters, E. I. Proynov, P. A. Pieniazek, Y. M. Rhee, J. Ritchie, E. Rosta, C. D. Sherrill, A. C. Simmonett, J. E. Subotnik, H. L. Woodcock III, W. Zhang, A. T. Bell and A. K. Chakraborty, Phys. Chem. Chem. Phys., 2006, 8, 3172 RSC.
- (a) Y. J. Chang, M. Watanabe, P.-T. Chou and T. J. Chow, Chem. Commun., 2012, 48, 726 RSC; (b) C. H. Huang and Y. J. Chang, Tetrahedron Lett., 2014, 55, 4938 CrossRef CAS.
- (a) H. Tian, X. Yang, R. Chen, R. Zhang, A. Hagfeldt and L. Sun, J. Phys. Chem. C, 2008, 112, 11203 Search PubMed; (b) R. Chen, G. Zhao, X. Yang, X. Jiang, J. Liu, H. Tian, Y. Gao, X. Liu, K. Han, M. Sun and L. Sun, J. Mol. Struct., 2008, 876, 102 CrossRef CAS.
- (a) G. Boschloo, L. Häggman and A. Hagfeldt, J. Phys. Chem. B, 2006, 110, 13144 CrossRef CAS PubMed; (b) Z. Ning, Y. Fu and H. Tian, Energy Environ. Sci., 2010, 3, 1170 RSC; (c) S. A. Haque, E. Palomares, B. M. Cho, A. N. M. Green, N. Hirita, D. R. Klug and J. R. Durrant, J. Am. Chem. Soc., 2005, 127, 3456 CrossRef CAS PubMed.
- M. K. Nazeeruddin, F. D. Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, B. Takeru and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 16835 CrossRef CAS PubMed.
- (a) J. E. Kroeze, N. Hirata, S. Koops, M. K. Nazeeruddin, L. Schmidt-Mende, M. Grätzel and J. R. Durrant, J. Am. Chem. Soc., 2006, 128, 16376 CrossRef CAS PubMed; (b) C. Kim, H. Choi, S. Kim, C. Baik, K. Song, M.-S. Kang, S. O. Kang and J. Ko, J. Org. Chem., 2008, 73, 7072 CrossRef CAS PubMed; (c) Z. Ning, Q. Zhang, H. Pei, J. Luan, C. Lu, Y. Cui and H. Tian, J. Phys. Chem. C, 2009, 113, 10307 CrossRef CAS.
- (a) J. Bisquert, J. Phys. Chem. B, 2002, 106, 325 CrossRef CAS; (b) Q. Wang, J. Moser and M. Grätzel, J. Phys. Chem. B, 2005, 109, 14945 CrossRef CAS PubMed; (c) R. Kern, R. Sastrawan, J. Ferber, R. Stangl and J. Luther, Electrochim. Acta, 2002, 47, 4213 CrossRef CAS.
- Z. Ning, Q. Zhang, W. Wu, H. Pei, B. Liu and H. Tian, J. Org. Chem., 2008, 73, 3971 CrossRef PubMed.
- (a) D. Kim, M. S. Kang, K. Song, S. O. Kang and J. Ko, Tetrahedron, 2008, 64, 10417 CrossRef CAS; (b) N. Cho, H. Choi, D. Kim, K. Song, M. S. Kang, S. O. Kang and J. Ko, Tetrahedron, 2009, 65, 6236 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental data of compound B–D, the 1H- and 13C-NMR spectra, theoretical calculation, UV/vis spectra, CV spectra, EIS spectra, and the devices incooperating DCA. See DOI: 10.1039/c5ra17898c |
| This journal is © The Royal Society of Chemistry 2015 |