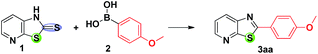
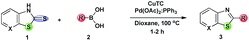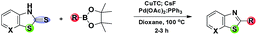An efficient desulfitative C–C cross coupling of fused thiazolidine-2-thione with boronic acids and boronic acid pinacol esters: formation of fused thiazoles†
Kandasamy Rajagurua,
Arumugam Mariappana,
Ramachandran Manjusria,
Shanmugam Muthusubramanian*a and
Nattamai Bhuvaneshb
aDepartment of Organic Chemistry, School of Chemistry, Madurai Kamaraj University, Madurai-625 021, India. E-mail: muthumanian2001@yahoo.com
bX-ray Diffraction Laboratory, Department of Chemistry, Texas A & M University, College Station, Texas 77842, USA
First published on 2nd October 2015
Abstract
An efficient Pd(0)-catalyzed Cu(I)-mediated desulfitative C–C cross-coupling of benzo-fused thiazolidine-2-thione with boronic acids under neutral Liebeskind–Srogl conditions is described. The desulfitative cross coupling of boronic acid pinacol esters has also been demonstrated with fused thiazolidine-2-thione under basic conditions to afford fused thiazoles with good to excellent yields.
Introduction
Desulfitative cross-coupling reactions involving alkyl thioethers, sulfones, and sulfonyls with organometallic reagents have been widely employed toward the synthesis of diverse molecules including important heterocyclic compounds.1 In this connection, palladium metal catalyzed – Cu(I) carboxylate mediated – Csp2–Csp2 cross coupling of boronic acids and various thioorganic compounds have been explored extensively under non-basic conditions for the construction of diverse cyclic and acyclic motifs.1,2 In this Liebeskind–Srogl cross coupling reaction, the soft Cu(I) carboxylate cofactor has high thiophilicity accelerating the desulfitative C–C cross couplings selectively.1f,3 The non-basic Liebeskind–Srogl cross coupling reaction has also gained attention, making considerable progress.1g,2c,4,5 The desulfitative cross-coupling of Sonogashira–Hagihara, Stille, and Suzuki–Miyaura as well as Mizoroki–Heck couplings of sulfonyl chlorides have been explored by Vogel and co-workers.6 Because of their stability, low toxicity, easy handling, and easy removal of degradable boron derived byproducts, “green” boronic acids7 are much superior to boronic esters as reactants.8 Currently, we are focusing on the development of methods for the synthesis of some functionalized heterocyclic compounds.9 In continuation, herein we report the Pd/Cu(I) mediated desulfitative carbon–carbon cross coupling for the synthesis of fused thiazole derivatives. Over the years, a variety of protocols have been developed for the rapid construction of benzo-fused or heteroaryl-fused thiazoles from prefunctionalized substrates.10 These highly functionalized thiazoles are found in many pharmaceutical agents11 and in some diverse materials with potential applications.12 The synthesis of substituted benzothiazoles has been achieved under Pd mediated Suzuki biaryl coupling of arylboronic acids with 2-halobenzothiazoles,13 while direct coupling of aryl bromides with benzothiazole14 is also known. Direct C–H arylation of heteroarenes via a denitrogenative and desulfitative mode of action has also been described.15,16 Gosmini and co-workers17 have elaborated the Co(II)-catalyzed synthesis of benzothiazole derivatives through coupling of aryl or benzylzinc reagents with methylsulfanyl-N-heteroarenes. It is pertinent to note that only very few reports are available towards the construction of heteroaryl fused thiazoles and that is the motive to undertake the present investigation.The starting materials for the present study, fused thiazolidine-2-thiones 1, have been prepared from cheap and readily available 2-haloanilines and highly reactive potassium ethyl xanthate.18 At first, the traditional Liebeskind–Srogl coupling reaction of 1 with arylboronic acid 2 with an effective thiophilic reagent – copper(I) thiophene-2-carboxylate (CuTC) and various palladium sources in the presence of phosphine ligands has been investigated (Scheme 1).
Results and discussion
The screening experiments have been started using a stoichiometric amount of 4-methoxyphenylboronic acid, Pd(dba)2 and PPh3 in the presence of a stoichiometric amount of CuTC. The temperature range studied was 50–100 °C, and the solvent tried was dioxane under a nitrogen atmosphere. This resulted in fused thiazoles as the desired product, but the conversion (22–42%) was not effective (Table 1, entries 1–3). Satisfactory results were obtained with Pd2(dba)3. When a combination of Pd(OAc)2 (10 mol%)–PPh3 (10 mol%)–RB(OH)2 (1.5 equiv.)–CuTC (2 equiv.) was tested at 100 °C with dioxane as the solvent, the reaction was complete in an hour under nitrogen giving the best result (89%) (Table 1, entry 8). Running the experiment with increased catalyst loading (20 mol%) or lowering the reaction temperature did not increase the yield. The effective conversion has been improved (64–89%, Table 1, entries 9–12) by a sacrificial increment of CuTC as well as arylboronic acid. This can be explained by the low thiophilicity of boron in the organoboron compounds and relatively low nucleophilic reactivity compared to CuTC.4c,19 The oxidation of Cu(I) cofactor has been avoided under the inert atmosphere, promoting the product yield.1c,2a,20 Phosphine free conditions (Table 1, entry 20) or extending the reaction time do not provide an encouraging result.| Entry | 2 (equiv.)/CuTC (equiv.) | Catalyst (mol%)/ligand (mol%) | Solvent | Time (h) | Temp (°C) | Yieldb (%) 3aa |
|---|---|---|---|---|---|---|
| a Experiments were performed in dioxane/THF (5 mL) under N2 atm.b Isolated yield.c No reaction.d L1 = PPh3; L2 = XPhos. | ||||||
| 1 | 1/1 | Pd(dba)2 (10)/L1 (5) | Dioxane | 16 | 50 | 22 |
| 2 | 1/1 | Pd(dba)2 (20)/L1 (10) | Dioxane | 12 | 50 | 34 |
| 3 | 1/1 | Pd(dba)2 (10)/XPhos (10) | Dioxane | 8 | 100 | 42 |
| 4 | 1.2/1 | Pd2(dba)3 CHCl3 (10)/L1 (10) | Dioxane | 5 | 80 | 55 |
| 5 | 1.5/2 | Pd2(dba)3 CHCl3 (10)/L1 (10) | Dioxane | 5 | 100 | 68 |
| 6 | 1.5/1 | Pd2(dba)3 CHCl3 (10)/L2 (10) | Dioxane | 3 | 100 | 52 |
| 7 | 1.5/2 | PdCl2 (10)/L2 (10) | Dioxane | 8 | 100 | 22 |
| 8 | 1.5/2 | Pd(OAc)2 (10)/L1 (10) | Dioxane | 1 | 100 | 89 |
| 9 | 1.5/2 | Pd(OAc)2 (10)/L1 (10) | THF | 1 | 100 | 72 |
| 10 | 1.5/2 | Pd(OAc)2 (20)/L1 (10) | Dioxane | 1 | 100 | 65 |
| 11 | 2/2 | Pd(OAc)2 (10)/L1 (5) | Dioxane | 1 | 100 | 64 |
| 12 | 2/3 | Pd(OAc)2 (10)/L1 (10) | Dioxane | 1 | 100 | 82 |
| 13 | 1.5/2 | Pd(OAc)2 (10)/L1 (10) | Dioxane | 1 | 130 | 32 |
| 14 | 1.5/2 | Pd(OAc)2 (5)/L1 (10) | THF | 1 | 100 | 52 |
| 15 | 1.2/1 | Pd(TFA)2 (10)/L1 (10) | Dioxane | 5 | 100 | 58 |
| 16 | 1.5/2 | Pd(TFA)2 (20)/L2 (10) | Dioxane | 5 | 100 | 66 |
| 17 | 1.5/2 | Pd(OAc)2 (10)/L1 (10) | Dioxane | 24 | rt | —c |
| 18 | 1/2 | Pd(MeCN)2Cl2 (10)/L1 (10) | Dioxane | 12 | 100 | 42 |
| 19 | 2/2 | Pd(MeCN)2Cl2 (10)/L2 (10) | Dioxane | 8 | 100 | 53 |
| 20 | 1.5/2 | Pd(OAc)2 (10 mol%) | Dioxane | 18 | 100 | c |
| 21 | 1.5/2 | Pd(PPh3)4 (10 mol%) | Dioxane | 8 | 100 | 61 |
Encouraged by this optimised condition, the substrate scope of the reaction has been explored by coupling a range of arylboronic acids and (E)-β-styrylboronic acids with 1 to obtain 3 in good yields and the derivatives synthesized are shown in Table 2.
| a Reaction conditions: thiazolidine-2-thione 1 (0.10 mmol), R–B(OH)2 2 (0.15 mmol), CuTC (0.20 mmol), Pd(OAc)2 (0.01 mmol, 10 mol%) and PPh3 (0.01 mmol, 10 mol%) in dioxane (5 mL) were heated at 100 °C for 1–2 h under N2 atm. |
|---|
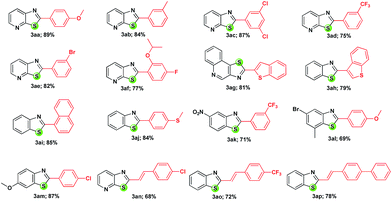 |
The homocoupling of boronic acids cannot be avoided resulting in biaryls due to the efficiency of the copper salt,21 though the conversion achieved is quite satisfactory. With sterically hindered arylboronic acids or some functionalized boronic acids or alkylboronic acids, the transformation was not successful under the optimized conditions. All the synthesized compounds have been characterized by NMR studies and further confirmed by single crystal X-ray analysis of 3ah (ref. 22) as shown in Fig. 1.
 | ||
| Fig. 1 X-ray crystal structures of 3ah and 3aw. Thermal ellipsoids are shown at the 50% probability level. | ||
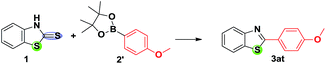
Having successfully effected the coupling with boronic acid, we wanted to test the efficacy of this protocol with organoboron pinacol esters. However, the neutral Liebeskind–Srogl desulfitative coupling reaction conditions are apparently not suitable in the case of organoboron pinacol esters, even with 3–5 equivalent of CuTC in the Pd(OAc)2/PPh3 system. The base is essential for the cross-coupling with organoboron pinacol esters. Earlier, Yu and Liebeskind8b have explored Pd(0)-catalyzed, Cu(I) carboxylate-mediated cross-coupling of thiol esters and B-alkyl-9-BBN under basic conditions. We started with oxygen bases such as Na2CO3, K2CO3, Cs2CO3 and K3PO4 to accelerate the activation of the organoboron pinacol ester in dioxane at 100 °C under a nitrogen atmosphere, which resulted in a poor yield with undesired side products. Fortunately, clean transformation has been achieved in the presence of a stoichiometric amount of KF with moderate yield, though switching over to CsF (2.0 equiv.) ended with the desired product in moderate to excellent yield (Table 3).
| Entry | 2′ (equiv.)/CuTC (equiv.) | Pd(OAc)2 (mol%)/PPh3 (mol%) | Base (equiv.) | Time (h) | Temp (°C) | Yieldb (%) 3ai |
|---|---|---|---|---|---|---|
| a Experiments were performed in dioxane (5 mL) under N2 atm.b Isolated yield.c No reaction. | ||||||
| 1 | 1.5/3 | 10/10 | Nil | 18 | 100 | —c |
| 2 | 1.5/5 | 10/10 | Nil | 18 | 100 | —c |
| 3 | 1.5/2 | 10/10 | Na2CO3 (1.0) | 18 | 100 | Trace |
| 4 | 1.5/2 | 10/10 | K2CO3 (1.0) | 12 | 100 | 12 |
| 5 | 1.5/2 | 10/10 | K3PO4 (2.0) | 8 | 100 | 28 |
| 6 | 1.5/2 | 10/10 | Cs2CO3 (1.0) | 8 | 100 | 42 |
| 7 | 1.5/2 | 10/10 | KF (2.0) | 5 | 100 | 61 |
| 8 | 1.5/2 | 10/10 | CsF (2.0) | 2 | 100 | 78 |
| 9 | 1.5/1 | 10/10 | CsF (2.0) | 8 | 100 | 34 |
| 10 | 1.5/2 | 10/10 | CsF (2.0) | 24 | rt | —c |
| 11 | 1.5/2 | 10/5 | CsF (2.0) | 2 | 100 | 58 |
| 12 | 1.5/2 | Pd(PPh3)4 (10) | CsF (2.0) | 4 | 100 | 43 |
| 13 | 1.5/2 | Pd(OAc)2 (10) | CsF (2.0) | 24 | 100 | —c |
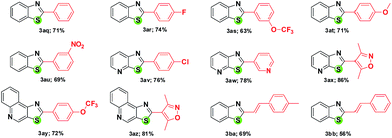
No reaction occurred in the absence of the ligand. The additional compounds added to the library of fused thiazole analogues through this protocol are shown in Table 4. It can be noted that under the optimized conditions, the coupling of thiazolidine-2-thione with the heteroaryl organoboron pinacol ester system seems to be more facile compared to arylboron pinacol esters. Interestingly, homocoupling is not a problem with boronic acid pinacolate ester.
| a Reaction conditions: thiazolidine-2-thione 1 (0.10 mmol), R–BPin 2′ (0.15 mmol), CuTC (0.20 mmol), Pd(OAc)2 (0.01 mmol, 10 mol%), PPh3 (0.01 mmol, 10 mol%) and CsF (0.20 mmol) in dioxane (5 mL) heated at 100 °C for 2–3 h under N2 atm. |
|---|
 |
The synthesized compounds were characterized by NMR studies and further confirmed by single crystal X-ray analysis of 3aw (ref. 26) as shown in Fig. 1.
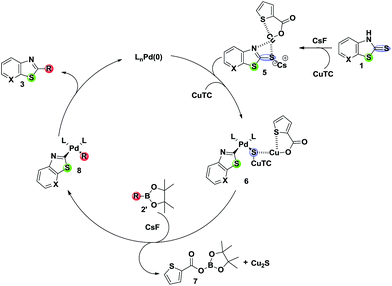
Based on the anticipated interaction between organosulfur and organoboron (thiophilic/borophilic) in the presence of Cu cofactors,1,2a,8b,23,24 a mechanism for the formation of 3 has been illustrated in Scheme 2. The proposed mechanism for the formation of 3 by the boranepinacol ester strategy starts with the Cu(I) thiolate species 5 obtained by the thione–thiol tautomerization,25 which undergoes oxidative addition with the Pd(0) catalyst and with an additional equivalent of CuTC cofactor to form an intermediate 6. In the transmetalation step, the fluoride accelerates the pinacolate deprotection from 2′ to yield 8 with extrusion of 7 and Cu2S. The subsequent reductive elimination results in the cross-coupled product 3.
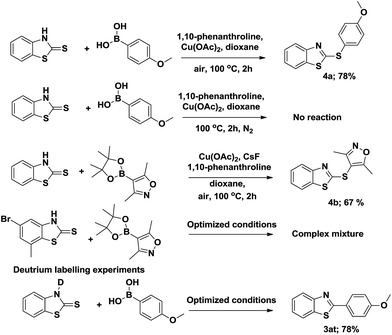
To support the proposed mechanism, some control experiments have been performed (Scheme 3). In the first experiment, a mixture of thiazolidine-2-thione and 4-methoxyphenylboronic acid (2 equiv.) (or boronic ester with CsF) was heated under aerobic conditions1g at 100 °C for 2 h in the presence of Cu(II) acetate (1.5 equiv.) and 1,10-phenanthroline (2 equiv.) in dioxane, which resulted in the carbon-sulfur cross-coupled product 4a. This reaction does not proceed under nitrogen atmosphere indicating that the reaction may go via disulfide formation. Under the optimized conditions, Suzuki active bromo substituted thiazolidine-2-thione and 4-methoxyphenylboronic acid (2 equiv.), again yielded the anticipated thiazole derivative without traces of the traditional Suzuki coupled product (Table 2), while with boronic acid pinacol esters, a complex mixture was observed with the bromo substituted thiazolidine-2-thione system. Finally, the reaction of N-deuterium substituted aryl thiazolidine-2-thione and 4-methoxyphenylboronic acid under the same conditions furnished the benzothiazole with no deuterium incorporation, proving no hydrogen exchange occurs in the mechanism. From the above observations, a chemoselective desulfitative coupling occurred at C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and in no case was the C–S–C was affected to yield fused hetero benzothiazoles.
S and in no case was the C–S–C was affected to yield fused hetero benzothiazoles.
In addition, under the optimized conditions the same strategy has also been tested with a controlled amount of water and under normal conditions in the absence of CuTC as well, but the desired product was not achieved (Table S1 see ESI†).
Conclusions
In summary, we have demonstrated a Pd(0)/Cu(I)-mediated desulfitative C–C cross-coupling of boronic acids with fused thiazolidine-2-thione under neutral conditions. We have also performed desulfitative coupling under basic conditions with thiazolidine-2-thione and organoboron pinacol esters resulting in C–C bond formation. This strategy involves mild reaction conditions towards the synthesis of diversely substituted benzo-fused thiazoles in good to excellent yields.Experimental section
General remarks
Nuclear magnetic resonance (1H and 13C NMR) spectra were recorded on a 300 MHz spectrometer in CDCl3 using TMS as an internal standard. Chemical shifts are reported in parts per million (δ), coupling constants (J values) are reported in Hertz (Hz) and spin multiplicities are indicated by the following symbols: s (singlet), d (doublet), t (triplet), m (multiplet). 13C NMR spectra were routinely run with broadband decoupling. Pre coated silica gel on aluminium plates (Merck) were used for TLC analysis with a mixture of petroleum ether (60–80 °C) and ethyl acetate as eluent. Elemental analyses were performed on a Perkin Elmer 2400 Series II Elemental CHNS analyser. Mass spectra were recorded in an LCQ Fleet mass spectrometer, Thermo Fisher Instruments Limited, US. Electrospray ionisation mass spectrometry (ESI-MS) analysis was performed in the positive ion and negative ion mode on a liquid chromatography ion trap.General procedure
Neutral conditions. To a stirred solution of thiazolidine-2-thione 1 (0.10 mmol) in dioxane (5 mL), R–B(OH)2 2 (0.15 mmol), CuTC (0.20 mmol), Pd(OAc)2 (0.01 mmol, 10 mol%) and PPh3 (0.01 mmol, 10 mol%) were added in a reaction vessel, and the reaction mixture heated at 100 °C for 1 h under N2 atm. The completion of the reaction was monitored by TLC. After cooling to room temperature, the reaction mixture was filtered through Celite. Then, the filtrate was washed with a saturated solution of NH4Cl (3 × 10 mL) and extracted with EtOAc (2 × 20 mL). The combined organic layers were dried with anhydrous Na2SO4 and concentrated in vacuo. The residue was then purified by column chromatography on silica gel (hexane–ethyl acetate 9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield arylthiazole 3.
1) to yield arylthiazole 3.
Basic conditions. To a stirred solution of thiazolidine-2-thione 1 (0.10 mmol) in dioxane (5 mL), R–BPin 2′ (0.15 mmol), CuTC (0.20 mmol), Pd(OAc)2 (0.01 mmol, 10 mol%), PPh3 (0.01 mmol, 10 mol%) and CsF (0.20 mmol) were added in a reaction vessel, and the reaction mixture heated at 100 °C for 2 h under N2 atm. The completion of the reaction was monitored by TLC. After cooling to room temperature, the reaction mixture was filtered through Celite. Then, the filtrate was washed with a saturated solution of NH4Cl (3 × 10 mL) and extracted with EtOAc (2 × 20 mL). The combined organic layers were dried with anhydrous Na2SO4 and concentrated in vacuo. The residue was then purified by column chromatography on silica gel (hexane–ethyl acetate 9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield aryl thiazole 3a.
1) to yield aryl thiazole 3a.
Acknowledgements
The authors thank DST, New Delhi for assistance under the IRHPA program for the NMR facility. K. R. gratefully acknowledges the award of a Junior Research Fellowship and financial support from CSIR (Grant No. 02(0061)/12/EMR-II), New Delhi.Notes and references
- (a) I. Erdelmeier and H. J. Gais, J. Am. Chem. Soc., 1989, 111, 1125 CrossRef CAS; (b) J. Srogl, G. D. Allred and L. S. Liebeskind, J. Am. Chem. Soc., 1997, 119, 12376 CrossRef CAS; (c) L. S. Liebeskind and J. Srogl, Org. Lett., 2002, 4, 979 CrossRef CAS PubMed; (d) K. Itami, D. Yamazaki and J. I. Yoshida, J. Am. Chem. Soc., 2004, 126, 15396 CrossRef CAS PubMed; (e) J. M. Villalobos, J. Srogl and L. S. Liebeskind, J. Am. Chem. Soc., 2007, 129, 15734 CrossRef CAS PubMed; (f) H. Prokopcova and C. O. Kappe, Angew. Chem., Int. Ed., 2009, 48, 2276 CrossRef CAS PubMed; (g) N. Arshad, J. Hashim and C. O. Kappe, J. Org. Chem., 2009, 74, 5118 CrossRef CAS PubMed.
- (a) L. S. Liebeskind and J. Srogl, J. Am. Chem. Soc., 2000, 122, 11260 CrossRef CAS; (b) K. Deres, C. H. Schroder, A. Paessens, S. Goldmann, H. J. Hacker, O. Weber, T. Kraemer, U. Niewoehner, U. Pleiss, J. Stoltefuss, E. Graef, D. Koletzki, R. N. A. Masantschek, A. Reimann, R. Jaeger, R. Grob, B. Beckermann, K.-H. Schlemmer, D. Haebich and R. H. Waigmann, Science, 2003, 299, 893 CrossRef CAS PubMed; (c) A. Lengar and C. O. Kappe, Org. Lett., 2004, 6, 771 CrossRef CAS PubMed.
- (a) C. Savarin and L. S. Liebeskind, Org. Lett., 2001, 3, 2149 CrossRef CAS PubMed; (b) C. Kusturin, L. S. Liebeskind, H. Rahman, K. Sample, B. Schweitzer, J. Srogl and W. L. Neumann, Org. Lett., 2003, 5, 4349 CrossRef CAS PubMed.
- (a) Z. Zhang and L. S. Liebeskind, Org. Lett., 2006, 8, 4331 CrossRef CAS PubMed; (b) H. Prokopcova and C. O. Kappe, Adv. Synth. Catal., 2007, 349, 448 CrossRef CAS PubMed; (c) H. Prokopcova and C. O. Kappe, J. Org. Chem., 2007, 72, 4440 CrossRef CAS PubMed.
- (a) S. Silva, B. Sylla, F. Suzenet, A. Tatibouet, A. P. Rauter and P. Rollin, Org. Lett., 2008, 10, 853 CrossRef CAS PubMed; (b) V. P. Mehta, A. Sharma and E. V. der Eycken, Org. Lett., 2008, 10, 1147 CrossRef CAS PubMed; (c) X. Guinchard and E. Roulland, Org. Lett., 2009, 11, 4700 CrossRef CAS PubMed; (d) Z.-F. Yan, Z.-J. Quan, Y.-X. Da, Z. Zhang and X.-C. Wang, Chem. Commun., 2014, 50, 13555 RSC.
- (a) S. R. Dubbaka and P. Vogel, J. Am. Chem. Soc., 2003, 125, 15292 CrossRef CAS PubMed; (b) S. R. Dubbaka and P. Vogel, Adv. Synth. Catal., 2004, 346, 1793 CrossRef CAS PubMed; (c) S. R. Dubbaka and P. Vogel, Org. Lett., 2004, 6, 95 CrossRef CAS PubMed; (d) S. R. Dubbaka and P. Vogel, Angew. Chem., Int. Ed., 2005, 44, 7674 CrossRef CAS PubMed; (e) P. Vogel, M. Turks, L. Bouchez, D. Markovic, A. V. Alvarez and J. A. Sordo, Acc. Chem. Res., 2007, 40, 931 CrossRef CAS PubMed.
- (a) R. J. Weir Jr and R. S. Fisher, Toxicol. Appl. Pharmacol., 1972, 23, 351 CrossRef CAS; (b) C. H. Linden, A. H. Hall and K. W. Kulig, J. Toxicol., Clin. Toxicol., 1986, 24, 269 CrossRef CAS; (c) D. G. Hall, in Boronic Acids Preparation and Applications in Organic Synthesis and Medicine, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005, p. 1 Search PubMed.
- (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (b) Y. Yu and L. S. Liebeskind, J. Org. Chem., 2004, 69, 3554 CrossRef CAS PubMed; (c) A. J. J. Lennox and G. C. L. Jones, Chem. Soc. Rev., 2014, 43, 412 RSC.
- (a) K. Rajaguru, R. Suresh, A. Mariappan, S. Muthusubramanian and N. Bhuvanesh, Org. Lett., 2014, 16, 744 CrossRef CAS PubMed; (b) R. Suresh, S. Muthusubramanian, R. Senthil Kumaran and G. Manickam, Asian J. Org. Chem., 2014, 3, 604 CrossRef CAS PubMed; (c) A. Mariappan, K. Rajaguru, S. Muthusubramanian and N. Bhuvanesh, Tetrahedron Lett., 2015, 56, 338 CrossRef CAS PubMed.
- (a) D. S. Bose and M. Idrees, J. Org. Chem., 2006, 71, 8261 CrossRef CAS PubMed; (b) H.-Q. Do and O. Daugulis, J. Am. Chem. Soc., 2007, 129, 12404 CrossRef CAS PubMed; (c) N. K. D. Riley and Y. A. Jackson, Tetrahedron, 2007, 63, 10276 CrossRef PubMed; (d) K. Inamoto, C. Hasegawa, K. Hiroya and T. Doi, Org. Lett., 2008, 10, 5147 CrossRef CAS PubMed; (e) L. L. Joyce and R. A. Batey, Org. Lett., 2009, 11, 2792 CrossRef CAS PubMed; (f) D. Ma, S. Xie, P. Xue, X. Zhang, J. Dong and Y. Jiang, Angew. Chem., Int. Ed., 2009, 48, 4222 CrossRef CAS PubMed; (g) W.-P. Hu, Y. K. Chen, C. C. Liao, H. S. Yu, Y. M. Tsai, S. M. Huang, F. Y. Tsai, H. C. Shen, L. S. Chang and J. J. Wang, Bioorg. Med. Chem., 2010, 18, 6197 CrossRef CAS PubMed; (h) K. Inamoto, C. Hasegawa, J. Kawasaki, K. Hiroya and T. Doi, Adv. Synth. Catal., 2010, 352, 2643 CrossRef CAS PubMed; (i) V. A. Kozlov, D. V. Aleksanyan, Y. V. Nelyubina, K. A. Lyssenko, V. Petrovskii, I. L. Odinets, A. A. Vasilev and I. L. Odinets, Organometallics, 2011, 30, 2920 CrossRef CAS; (j) H. Wang, L. Wang, J. Shang, X. Li, H. Wang, J. Gui and A. Lei, Chem. Commun., 2012, 48, 76 RSC; (k) Y. Cheng, J. Yang, Y. Qu and P. Li, Org. Lett., 2012, 14, 98 CrossRef CAS PubMed; (l) Y. Liao, H. Qi, S. Chen, P. Jiang, W. Zhou and G.-J. Deng, Org. Lett., 2012, 14, 6004 CrossRef CAS PubMed.
- (a) B. L. Mylari, E. R. Larson, T. A. Beyer, W. J. Zembrowski, C. E. Aldinger, M. F. Dee, T. W. Siegel and D. H. Singleton, J. Med. Chem., 1991, 34, 108 CrossRef CAS; (b) C. G. Mortimer, G. Wells, J.-P. Crochard, E. L. Stone, T. D. Bradshaw, M. F. G. Stevens and A. D. Westwell, J. Med. Chem., 2006, 49, 179 CrossRef CAS PubMed; (c) S. Aiello, G. Wells, E. L. Stone, H. Kadri, R. Bazzi, D. R. Bell, M. F. G. Stevens, C. S. Matthews, T. D. Bradshaw and A. D. Westwell, J. Med. Chem., 2008, 51, 5135 CrossRef CAS PubMed; (d) J. Mondal, S. Sreejith, P. Borah and Y. Zhao, ACS Sustainable Chem. Eng., 2014, 2, 934 CrossRef CAS.
- (a) K. Serdons, C. Terwinghe, P. Vermaelen, K. V. Laere, H. Kung, L. Mortelmans, G. Bormans and A. Verbruggen, J. Med. Chem., 2009, 52, 1428 CrossRef CAS PubMed; (b) S. Ramurthy, M. Aikawa, P. Amiri, A. Costales, A. Hashash, J. M. Jansen, S. Lin, S. Ma, P. A. Renhowe, C. M. Shafer, S. Subramanian, L. Sung and J. Verhagen, Bioorg. Med. Chem. Lett., 2011, 21, 3286 CrossRef CAS PubMed; (c) M. M. M. Raposo, M. C. R. Castro, M. Belsley and A. M. C. Fonseca, Dyes Pigm., 2011, 91, 454 CrossRef CAS PubMed; (d) B. C. Lee, J. S. Kim, B. S. Kim, J. Y. Son, S. K. Hong, H. S. Park, B. S. Moon, J. H. Jung, J. M. Jeong and S. E. Kim, Bioorg. Med. Chem., 2011, 19, 2980 CrossRef CAS PubMed; (e) H. Amada, Y. Sekiguchi, N. Ono, Y. Matsunaga, T. Koami, H. Asanuma, F. Shiozawa, M. Endo, A. Ikeda, M. Aoki, N. Fujimoto, R. Wada and M. Sato, Bioorg. Med. Chem. Lett., 2012, 22, 2024 CrossRef CAS PubMed.
- (a) V. J. Majo, J. Prabhakaran, J. J. Mann and J. S. D. Kumar, Tetrahedron Lett., 2003, 44, 8535 CrossRef CAS PubMed; (b) Y. Heo, Y. S. Song, B. T. Kim and J. N. Heo, Tetrahedron Lett., 2006, 47, 3091 CrossRef CAS PubMed.
- D. Alagille, R. M. Baldwin and G. D. Tamagnan, Tetrahedron Lett., 2005, 46, 1349 CrossRef CAS PubMed.
- (a) M. Zhang, S. Zhang, M. Liu and J. Cheng, Chem. Commun., 2011, 47, 11522 RSC; (b) R. Chen, S. Liu, X. Liu, L. Yang and G.-J. Deng, Org. Biomol. Chem., 2011, 9, 7675 RSC; (c) B. Liu, J. Li, F. Song and J. You, Chem.–Eur. J., 2012, 18, 10830 CrossRef CAS PubMed; (d) M. Wang, D. Li, W. Zhou and L. Wang, Tetrahedron, 2012, 68, 1926 CrossRef CAS PubMed.
- (a) X. Yu, X. Li and B. Wan, Org. Biomol. Chem., 2012, 10, 7479 RSC; (b) C. M. R. Volla and P. Vogel, Angew. Chem., Int. Ed., 2008, 47, 1305 CrossRef PubMed; (c) C. M. R. Volla, D. Markovic, S. R. Dubbaka and P. Vogel, Eur. J. Org. Chem., 2009, 6281 CrossRef CAS PubMed.
- J.-M. Begouin, M. Rivard and C. Gosmini, Chem. Commun., 2010, 46, 5972 RSC.
- M. E. Layton and M. J. Kelly, 1,3-Benzothiazol-2(3H)-ones and [1,3]thiazolo[5,4-b]pyridin-2(1H)-ones as positive allosteric modulators of mGluR2 and their preparation and use for the treatment of neurological and psychiatric disorders, U. S. PCT Int. Appl., WO 2011137046A1, 3rd November 2011.
- L. Wang, W. He and Z. Yu, Chem. Soc. Rev., 2013, 42, 599 RSC.
- (a) F.-A. Alphonse, F. Suzenet, A. Keromnes, B. Lebret and G. Guillaumet, Synlett, 2002, 447 CrossRef CAS; (b) M. Egi and L. S. Liebeskind, Org. Lett., 2003, 5, 801 CrossRef CAS PubMed; (c) F.-A. Alphonse, F. Suzenet, A. Keromnes, B. Lebret and G. Guillaumet, Org. Lett., 2003, 5, 803 CrossRef CAS PubMed; (d) R. Wittenberg, J. Srogl, M. Egi and L. S. Liebeskind, Org. Lett., 2003, 5, 3033 CrossRef CAS PubMed.
- (a) S. Zhang, D. Zhang and L. S. Liebeskind, J. Org. Chem., 1997, 62, 2312 CrossRef CAS; (b) A. S. Demir, O. Reis and M. Emrullahoglu, J. Org. Chem., 2003, 68, 10130 CrossRef CAS PubMed.
- ESI†.
- (a) C. Savarin, J. Srogl and L. S. Liebeskind, Org. Lett., 2001, 3, 91 CrossRef CAS; (b) C. L. Kusturin, L. S. Liebeskind and W. L. Neumann, Org. Lett., 2002, 4, 983 CrossRef CAS PubMed; (c) H. Yang, H. Li, R. Wittenberg, M. Egi, W. Huang and L. S. Liebeskind, J. Am. Chem. Soc., 2007, 129, 1132 CrossRef CAS PubMed; (d) W. Jin, W. Du, Q. Yang, H. Yu, J. Chen and Z. Yu, Org. Lett., 2011, 13, 4272 CrossRef CAS PubMed.
- (a) E. S. Raper, Coord. Chem. Rev., 1994, 129, 91 CrossRef CAS; (b) S. C. Davies, M. C. Durrant, D. L. Hughes, K. Leidenberger, C. Stapper and R. L. Richards, J. Chem. Soc., Dalton Trans., 1997, 2409 RSC; (c) C. Wycliff, D. S. Bharathi, A. G. Samuelson and M. Nethaji, Polyhedron, 1999, 18, 949 CrossRef CAS.
- S. Stoyanov, I. Petkov, L. Antonov and T. Stoyanova, Can. J. Chem., 1990, 68, 1482 CrossRef CAS.
- ESI†.
- H. Goog, D. Won, J. Yong, S. Mi, S. Hee, S. Youn, J. Seok, K. Oh and H. Don, New Electron Transport Material and Organic Electroluminescent Device Using The Same, Patent WO2013180376 A1, 5th December 2013.
- X.-L. Yang, C.-M. Xu, S.-M. Lin, J.-X. Chen, J.-C. Ding, H.-Y. Wu and W.-K. Su, J. Braz. Chem. Soc., 2010, 21, 37 CrossRef CAS.
- N. Park, Y. Heo, M. R. Kumar, Y. Kim, K. H. Song and S. Lee, Eur. J. Org. Chem., 2012, 1984 CrossRef CAS PubMed.
- S.-J. Choi, H. J. Park, S. K. Lee, S. W. Kim, G. Han and H.-Y. P. Choo, Bioorg. Med. Chem., 2006, 14, 1229 CrossRef CAS PubMed.
- L. W. Wattenberg, M. A. Page and J. L. Leong, Cancer Res., 1968, 28, 2539 CAS.
- D. X. Cao, Q. Fang, D. Wang, Z.-Q. Liu, G. Xue, G.-B. Xu and W.-T. Yu, Eur. J. Org. Chem., 2003, 3628 CrossRef CAS PubMed.
- X. Li, T. Yuan, Y. Yang and J. Chen, Tetrahedron, 2014, 70, 9652 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C NMR & mass spectra of all the synthesized compounds. CCDC 1059158 and 1059159. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra17827d |
| This journal is © The Royal Society of Chemistry 2015 |