Metal free synthesis of morpholine fused [5,1-c] triazolyl glycoconjugates via glycosyl azido alcohols†
Kunj B. Mishra,
Somesh Shashi and
Vinod K. Tiwari*
Department of Chemistry, Centre of Advanced Study, Faculty of Science, Banaras Hindu University, Varanasi-221005, India. E-mail: Tiwari_chem@yahoo.co.in
First published on 28th September 2015
Abstract
A series of diverse glycosyl 1,2-azido alcohols, obtained from readily available carbohydrates, were converted to structurally varied rare and novel sugar derived morpholine fused [5,1-c]-triazoles via a one-pot strategy. After incorporating a propargyl functionality at the hydroxyl group of the sugar derived 1,2-azido alcohols, the resulting in situ generated azido–alkyne affords numerous C- and O-glycosyl bicyclic ring systems with medicinal value via a metal free cycloaddition reaction. The structures of all the developed molecules have been elucidated using 1H NMR, 13C NMR, IR and MS spectroscopy.
1. Introduction
The design and development of medium-sized organic scaffolds and fused heterocyclic systems ‘found in numerous pharmaceutical molecules’ are attractive to chemists for the synthesis of novel mechanism based drugs and to improve the therapeutic efficacy of clinical medicines by adjoining these scaffolds.1 1,2-Azido alcohols have been considered as important precursors for the synthesis of such medium sized heterocyclic systems which can show excellent biological activities.2 1,2-Azido alcohols conjugated with carbohydrates pave the way for the creation of a large number of fused bicyclic molecules linked with biocompatible sugar, which is responsible for the enhancement of the biological action of such systems. Hence the synthesis of glycoconjugate azido alcohols and their application in organic chemistry is an increasing demand for the advancement of modern drug discovery. Morpholine, has been found to be an excellent pharmacophore in medicinal chemistry. A number of drugs including morpholine as a constituent are available in the market.3 Therefore, many new approaches toward the synthesis of morpholine derivatives have been reported in the literature.4The 1,2,3-triazole core, which is part of a significant class of biologically active nitrogen compounds, exhibits a number of important biological properties such as antibacterial, anticancer, antivirus, antituberculosis, anti-HIV, antitumor, and glycosidase inhibition.5–8 Moreover, 1,2,3-triazoles have found wide industrial applications as dyes, agrochemicals, corrosion inhibitors, and photostabilizers.9 Hence, the synthesis of the 1,2,3-triazole nucleus or its conjugation with a key part of a medicine has been the subject of ever growing synthetic efforts.
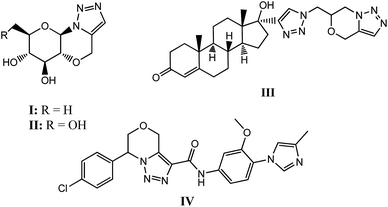
The Huisgen dipolar cycloaddition of alkynes with organic azides is a conventional path for the synthesis of 1,2,3-triazoles but is limited due to the formation of a regioisomeric mixture in the form of 1,4- and 1,5-isomers as products.10 The CuAAC reaction represents an extremely powerful tool for the rapid coupling of organic azides and terminal alkynes and produces 1,4-disubstituted 1,2,3-triazoles solely.11 Likewise, the ruthenium catalyzed azide–alkyne cycloaddition (RuAAC) frequently gives the opposite 1,5-regioisomer of 1,2,3-triazole; although expensive ruthenium complexes are required as catalysts.12 Furthermore, it is realized that the metal free intramolecular azide–alkyne cycloaddition is an economically favourable and easy to perform which affords the regioselective 1,5-disubstituted 1,2,3-triazoles fused with hetero and carbocyclic systems of medicinal value. Molecules having such scaffolds exhibit efficient and precious biological responses including α-glucosidase enzyme (I), α-galactosidase (II), and active against Alzheimer disease (IV) (Fig. 1).2 It would thus be interesting to synthesize molecules possessing these moieties in conjugation with carbohydrates in fused form and evaluate their biological properties. Presently the world drug index contains numerous drugs having this structural feature in different forms including scaffold, side-chain, fused-ring, etc.13
Herein, we report the synthesis of novel C- and O-glycosylated morpholine fused 1,5-triazoles from diverse glycosyl azido alcohols using a metal-free intramolecular thermal azide–alkyne cycloaddition reaction.
2. Results and discussion
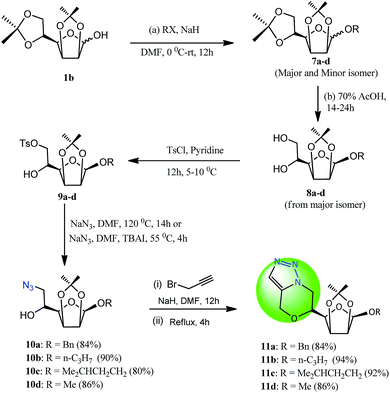
Our synthetic investigation begins with readily available monosaccharides (D-glucose, D-mannose, D-galactose, D-ribose, D-xylose, etc.), which after processing with a number of high yielding steps of protections and modifications afford diverse orthogonally protected sugars 1a–e. The 1,2:5,6-di-O-isopropylidene-α-D-glucofuranose 1a and 2,3:5,6-di-O-isopropylidene-D-mannofuranose 1b were taken as starting materials for the synthesis of diverse glucosidal and mannosidal furanosyl 1,2-azido alcohols. First of all, compound 1a was subjected to a substitution reaction with diverse organic halides (benzyl bromide, iodomethane, iodoethane, isopentyl bromide, and chloropropane) using basic conditions in dry DMF under argon atmosphere to afford their respective O-substituted derivatives 2a–e. Compounds 2a–e were further converted to their corresponding diols 3a–e via selective 5,6-isopropylidene deprotection using mild acidic conditions. Compounds 3a–e were next reacted with tosyl chloride in dry pyridine at 5–10 °C for 12 hours to afford their selective tosyl derivatives 4a–e. The subsequent azidation of all tosylated sugars with sodium azide in dry DMF at 115 °C employing anhydrous condition resulted in their respective deoxy-azido sugars 5a–e in excellent yields (Scheme 1, Table 1).14 | ||
| Scheme 1 Synthesis of morpholine-fused [5,1-c]-triazoles (5a–e) from D-glucose via the azido alcohol route. | ||
Once the synthesis of azido-alcohols 5a–e was achieved, we next attempted the metal free azide–alkyne intramolecular cycloaddition reaction. We utilized all the developed azido alcohols for the synthesis of the desired morpholine fused [5,1-c]-triazoles via O-propargylation at the secondary hydroxyl group of the 1,2-azido alcohols using strong base (NaH) in dry DMF at 0 °C–rt which gave an azido–alkyne as the intermediate. After quenching of the remaining NaH by adding a few drops of water to the reaction mixture and without isolating the azido–alkyne intermediate, the reaction system was subjected to the thermal intramolecular azide–alkyne cyclization.
In the reaction optimization study, we reacted 5a (1.0 equiv.) with propargyl bromide (1.3 equiv.) and NaH (3.0 equiv.) in dry DMF (8 ml) for 12 h, then after the quenching process, the reaction mixture was heated at 110 °C for the next 4 hours. Subsequent purification using column chromatography gave compound 6a in excellent yields (92%).
The structure of compound 6a was deduced from extensive spectral studies (IR, NMR, and MS). The 1H NMR spectrum of compound 6a exhibited a singlet of one proton observed at δ 7.39 assigned to the triazole-H proton. In addition to other signals, the appearance of a double doublet at δ 4.92 is attributed to the characteristic OCHA proton of the triazolo morpholine ring while the expected other characteristic double doublet for the corresponding OCHB proton of the morpholine ring was found merged with benzylic protons which finally confirmed the occurrence of the thermal cyclization. A doublet at δ 5.87 (3.6 Hz) confirmed the presence of the anomeric proton while multiplets from δ 4.68 to δ 4.46 and δ 4.19 to 4.08 displayed the remaining carbohydrate protons. Two signals for six isopropylidene protons are observed at δ 1.43 and δ 1.26. 13C NMR of compound 6a showed two resonances at δ 129.6 and δ 127.8 corresponding to triazole-carbons. The molecular ion peak at 374 (M + H+) is evidence for the synthesis of compound 6a.
Similar chemistry has been implemented with 2,3:5,6-di-O-isopropylidene-D-mannofuranose. In this case, 1-O-substituted derivatives were obtained as a mixture of anomers where β-isomers 7a–d were isolated as the major constituent which were used for the subsequent formation of diols (8a–d), tosyl derivatives 9a–d, azido alcohols 10a–d and finally morpholine-fused [5,1-c]-triazoles 11a–d (Scheme 2). We further extended our investigation to the synthesis of another series of azido alcohols and their utilization in the synthesis of morpholine fused triazoles.
Therefore, the analogues 1a–f were treated with epichlorohydrin under basic medium in dry DMF affording an excellent yield of glycosyl epoxides 12a–e along with non sugar derivative 12f. These glycosyl epoxides underwent regioselective azidation using a NaN3 in EtOH–H2O system at 65 °C in the presence of ammonium chloride affording O-glycosylated azido alcohols 13a–e and N-azido alcohol 13f (Scheme 3). The azido alcohols 13a–f thus obtained, on base-mediated propargylation using propargyl bromide in DMF followed by subsequent metal free azide–alkyne cycloaddition under thermal condition successfully afforded the desired morpholine fused [5,1-c]-triazoles (14a–f) in good to excellent yields (Scheme 4, Table 1). The structure of all compounds was deduced from extensive spectral studies (IR, NMR, and MS).
Although a detailed investigation is required to confirm the mechanism, we envisaged that the reaction may first involve base-prompted propargylation of azido alcohols (5, 10, and 13) followed by the metal-free thermal cycloaddition of the intermediate azido–alkyne. The intramolecular cycloaddition to afford the morpholine-fused 1,2,3-triazole is possible via two different routes. Because of the strain free environment, cyclization via path-I is more feasible which results in the regioselective formation of the desired morpholine-fused 1,5-triazoles (6, 11, and 14). Alternatively, in the case of cyclization via path II, attack of C2 of the terminal alkyne on the N1-atom of azide, simultaneous with attack of the N3 of azide on C1 of alkyne is not feasible, possibly due to the highly steric and strained environment in the small cavity of 1,4-disubstituted triazolyl macrocycle (Scheme 5).15
In summary, we have developed diverse novel morpholine-fused [5,1,-c] triazolyl glycoconjugates using an efficient and high yielding practical methodology. Three different series of novel glycosyl azido alcohols have been developed which have been utilized as starting materials for the synthesis of triazolo morpholines. The protocol exhibits a wide substrate scope, uses cheap and readily available reagents, is easy to perform, and is a high yielding metal free reaction that creates rare and biologically relevant heterocyclic molecules.
3. Experimental
3.1. General methods
All of the reactions were executed in anhydrous solvents (where required) under an argon atmosphere in glassware oven dried for one hour at 100 °C. All reagents and solvents were of pure analytical grade. Thin layer chromatography (TLC) was performed on 60 F254 silica gel, pre-coated on aluminum plates and revealed with either a UV lamp (λmax = 254 nm) or a specific colour reagent (Dragendorff reagent or iodine vapours) or by spraying with methanolic-H2SO4 solution and subsequent charring by heating at 100 °C. 1H and 13C NMR were recorded at 300 and 75 MHz, respectively. Chemical shifts are given in ppm downfield from internal TMS; J values in Hz. Mass spectra were recorded using electrospray ionization mass spectrometry (ESI-MS). Infrared spectra were recorded as Nujol mulls in KBr plates.1,2-O-Isopropylidene-3-O-propyl-6-O-tosyl-α-D-glucofuranose (4b). A stirring solution of 1,2-O-isopropylidene-3-O-propyl-α-D-glucofuranose (1.34 g, 5.1 mmol) in pyridine was treated with p-toluene sulphonyl chloride (1.0 g, 5.1 mmol) at 0 °C under anhydrous conditions followed by stirring for 12 h at 5–10 °C to afford a viscous liquid (1.3 g, 62%); 1H NMR (300 MHz, CDCl3): δ 7.80 (d, J = 8.4 Hz, 1H), 7.35 (d, J = 7.8 Hz, 1H), 5.86 (d, J = 3.6 Hz, 1H), 4.53 (d, J = 3.6 Hz, 1H), 4.28–3.96 (m, 5H), 3.61–3.40 (m, 2H), 2.45 (s, 3H), 1.60–1.53 (m, 2H), 1.46, 1.30 (each s, 6H), 0.90 (t, J = 7.5 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 145.0, 132.5, 129.9, 128.1, 111.8, 105.0, 82.7, 82.0, 79.0, 72.2, 67.5, 26.6, 26.1, 22.8, 21.5, 10.4 ppm.
3-O-Isopentyl-1,2-O-isopropylidene-6-O-tosyl-α-D-glucofuranose (4c). A stirring solution of 3-O-isopentyl-1,2-O-isopropylidene-α-D-glucofuranose (1.6 g, 5.7 mmol) in pyridine was treated with p-toluene sulphonyl chloride (1.08 g, 5.7 mmol) at 0 °C under anhydrous conditions followed by stirring for 12 h at 10 °C to afford a viscous liquid (1.5 g, 60%); 1H NMR (300 MHz, CDCl3): δ 7.80 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 8.1 Hz, 1H), 5.85 (d, J = 3.6 Hz, 1H), 4.52 (d, J = 3.6 Hz, 1H), 4.25 (dd, J = 2.7 Hz, 9.9 Hz, 1H), 4.17–4.15 (m, 1H), 4.11–4.06 (m, 2H), 4.04 (d, J = 3.0 Hz, 1H), 3.68–3.45 (m, 2H), 2.88 (d, J = 5.7 Hz, 1H), 2.45 (s, 3H), 1.67–1.60 (m, 2H), 1.46–1.43 (m, 4H), 1.31 (s, 3H), 0.89 (d, J = 6.6 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 145.1, 132.6, 130.0, 128.1, 111.9, 105.0, 82.8, 81.9, 79.0, 72.2, 68.9, 67.5, 38.4, 26.7, 26.1, 24.8, 22.5, 22.3, 21.5 ppm.
1,2-O-Isopropylidene-3-O-methyl-6-O-tosyl-α-D-glucofuranose (4d). A stirring solution of 1,2-O-isopropylidene-3-O-methyl-α-D-glucofuranose (2.0 g, 8.5 mmol) in pyridine was treated with p-toluene sulphonyl chloride (1.62 g, 8.5 mmol) at 0 °C under anhydrous conditions followed by stirring for 12 h at 10 °C to afford a viscous liquid (1.97 g, 60%); 1H NMR (300 MHz, CDCl3): δ 7.77 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 7.8 Hz, 1H), 5.90 (d, J = 3.3 Hz, 1H), 4.60 (d, J = 3.6 Hz, 1H), 4.38 (dd, J = 4.8 Hz, 13.5 Hz, 1H), 4.17–4.10 (m, 4H), 3.90 (d, J = 1.8 Hz, 1H), 3.46 (s, 3H), 2.44 (s, 3H), 1.48, 1.32 (each s, 6H); 13C NMR (75 MHz, CDCl3): δ 144.9, 132.3, 129.8, 127.8, 111.6, 105.0, 84.0, 81.1, 79.1, 67.4, 67.4, 57.6, 26.5, 26.0, 21.3 ppm.
3-O-Ethyl-1,2-O-isopropylidene-6-O-tosyl-α-D-glucofuranose (4e). A stirring solution of 1,2-O-isopropylidene-3-O-ethyl-α-D-glucofuranose (0.80 g, 3.2 mmol) in pyridine was treated with p-toluene sulphonyl chloride (0.61 g, 3.2 mmol) at 0 °C under anhydrous conditions followed by stirring for 12 h at 5–10 °C to afford a viscous liquid (1.00 g, 78%); 1H NMR (300 MHz, CDCl3): δ 7.80 (d, J = 7.8 Hz, 1H), 7.35 (d, J = 7.8 Hz, 1H), 5.87 (d, J = 3.6 Hz, 1H), 4.53 (d, J = 3.6 Hz, 1H), 4.26 (dd, J = 2.7 Hz, 9.6 Hz, 1H), 4.18 (m, 1H), 4.12–4.06 (m, 2H), 4.00–3.97 (m, 1H), 3.74–3.51 (m, 2H), 2.94 (s, 1H), 2.45 (s, 3H), 1.46, 1.30 (each s, 6H), 1.19 (t, J = 6.6 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 145.1, 132.9, 130.0, 128.1, 111.8, 105.8, 82.6, 78.9, 78.4, 72.2, 67.5, 65.9, 26.7, 26.6, 21.5, 15.1 ppm.
Benzyl-2,3-O-isopropylidene-6-O-tosyl-β-D-mannofuranose (9a). A stirring solution of benzyl-2,3-O-isopropylidene-β-D-mannofuranose (2.70 g, 8.4 mmol) in pyridine was treated with p-toluene sulphonyl chloride (1.90 g, 8.4 mmol) at 0 °C under anhydrous conditions followed by stirring for 12 h at 10 °C to afford a white solid (1.96 g, 50%); 1H NMR (300 MHz, CDCl3): δ 7.82 (d, J = 8.1 Hz, 1H), 7.35–7.26 (m, 7H), 5.05 (s, 1H), 4.82 (dd, J = 4.2 Hz, 5.7 Hz, 1H), 4.64–4.56 (m, 2H), 4.41 (d, J = 11.7 Hz, 1H), 4.28–4.24 (m, 1H), 4.16–4.13 (m, 2H), 3.94 (dd, J = 3.6 Hz, 7.2 Hz, 1H), 2.68 (s, 1H), 2.43 (s, 3H), 1.42, 1.30 (each s, 6H); 13C NMR (75 MHz, CDCl3): δ 145.0, 132.8, 129.9, 128.5, 128.0, 112.8, 105.2, 84.7, 79.6, 78.2, 71.5, 69.0, 68.0, 25.7, 24.4, 21.5 ppm.
Propyl-2,3-O-isopropylidene-6-O-tosyl-β-D-mannofuranose (9b). A stirring solution of propyl-2,3-O-isopropylidene-β-D-mannofuranose (2.68 g, 10.2 mmol) in pyridine was treated with p-toluene sulphonyl chloride (1.9 g, 10.2 mmol) at 0 °C under anhydrous conditions followed by stirring for 12 h at 10 °C to afford a viscous liquid (2.6 g, 62%); 1H NMR (300 MHz, CDCl3): δ 7.82 (d, J = 8.1 Hz, 1H), 7.31 (d, J = 8.1 Hz, 1H), 4.96 (s, 1H), 4.81 (dd, J = 3.9 Hz, 5.7 Hz, 1H), 4.57 (d, J = 6.0 Hz, 1H), 4.32–4.16 (m, 3H), 3.91 (dd, J = 3.6 Hz, 7.5 Hz, 1H), 3.51 (d, J = 6.6 Hz, 15.6 Hz, 3H), 3.33–3.26 (m, 1H), 2.70 (s, 1H), 2.45 (s, 3H), 1.57–1.48 (m, 2H), 1.43, 1.30 (each s, 6H), 0.89 (t, J = 7.5 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 145.0, 132.8, 129.9, 128.0, 112.7, 105.9, 84.7, 79.7, 78.0, 71.5, 69.0, 68.1, 25.7, 24.4, 22.5, 21.5, 10.4 ppm.
Isopentyl-2,3-O-isopropylidene-6-O-tosyl-β-D-mannofuranose (9c). A stirring solution of isopentyl-2,3-O-isopropylidene-β-D-mannofuranose (3.28 g, 11.3 mmol) in pyridine was treated with p-toluene sulphonyl chloride (2.14 g, 11.3 mmol) at 0 °C under anhydrous conditions followed by stirring for 12 h at 5–10 °C to afford a white solid (2.75 g, 55%); 1H NMR (300 MHz, CDCl3): δ 7.81 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 8.1 Hz, 1H), 4.96 (s, 1H), 4.82–4.78 (m, 1H), 4.55 (d, J = 6.0 Hz, 1H), 4.31 (d, J = 7.5 Hz, 1H), 4.17–4.15 (m, 2H), 3.89 (dd, J = 3.6 Hz, 7.2 Hz, 1H), 3.60 (dd, J = 6.9 Hz, 16.5 Hz, 1H), 3.36 (dd, J = 6.3 Hz, 16.2 Hz, 1H), 2.67 (d, J = 5.4 Hz, 1H), 2.45 (s, 3H), 1.69–1.58 (m, 2H), 1.48 (m, 1H), 1.43, 1.30 (each s, 6H), 0.88 (d, J = 4.8 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 145.0, 132.6, 129.9, 128.0, 112.7, 106.0, 84.7, 79.7, 78.1, 71.6, 68.2, 65.8, 38.0, 25.7, 24.8, 24.4, 22.4, 22.3, 21.5 ppm.
Methyl-2,3-O-isopropylidene-6-O-tosyl-β-D-mannofuranose (9d). A stirring solution of methyl-2,3-O-isopropylidene-β-D-mannofuranose (1.96 g, 8.3 mmol) in pyridine was treated with p-toluene sulphonyl chloride (1.5 g, 8.3 mmol) at 0 °C under anhydrous conditions followed by stirring for 12 h at 10 °C to afford a viscous liquid (2.0 g, 65%); 1H NMR (300 MHz, CDCl3): δ 7.82 (d, J = 7.5 Hz, 1H), 7.35 (d, J = 7.5 Hz, 1H), 4.86–4.80 (m, 2H), 4.54 (d, J = 5.7 Hz, 1H), 4.31 (d, J = 7.5 Hz, 1H), 4.19–4.17 (m, 2H), 3.91 (s, 1H), 3.26 (s, 3H), 2.68 (s, 1H), 2.44 (s, 3H), 1.49, 1.30 (each s, 6H).
6-Azido-6-deoxy-1,2-O-isopropylidene-3-O-propyl-α-D-glucofuranose (5b). Reaction of compound 4b (1.34 g, 3.2 mmol) with NaN3 (0.624 g, 9.6 mmol) in DMF at 115 °C afforded compound 5b. Oily liquid (0.81 g, 88% yield); IR (KBr) cm−1: 3453, 2964, 2935, 2878, 2103, 1633, 1455, 1080; 1H NMR (300 MHz, CDCl3): δ 5.91 (d, J = 3.6 Hz, 1H), 4.57 (d, J = 3.6 Hz, 1H), 4.10–4.00 (m, 3H), 3.66–3.42 (m, 4H), 2.74 (s, 1H), 1.65–1.58 (m, 2H), 1.49, 1.32 (each s, 6H), 0.94 (t, J = 7.5 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 111.8, 105.1, 82.8, 81.9, 79.7, 71.9, 68.9, 54.5, 26.7, 26.1, 22.8, 10.4 ppm.
6-Azido-6-deoxy-3-O-isopentyl-1,2-O-isopropylidene-α-D-glucofuranose (5c). Reaction of compound 4c (1.5 g, 3.3 mmol) with NaN3 (0.658 g, 10.0 mmol) in DMF at 115 °C afforded compound 5c. Oily liquid (0.98 g, 92% yield); IR (KBr) cm−1: 3472, 2958, 2872, 2104, 1711, 1633, 1466, 1081; 1H NMR (300 MHz, CDCl3): δ 5.90 (d, J = 3.6 Hz, 1H), 4.57 (d, J = 3.6 Hz, 1H), 4.10–3.98 (m, 3H), 3.73–3.42 (m, 4H), 2.64 (s, 1H), 1.72–1.63 (m, 2H), 1.49 (m, 4H), 1.32 (s, 3H), 0.91 (d, J = 6.6 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 111.9, 105.1, 82.9, 81.9, 79.8, 68.9, 68.7, 54.5, 38.4, 26.7, 26.2, 24.9, 22.5, 22.3 ppm.
6-Azido-6-deoxy-1,2-O-isopropylidene-3-O-methyl-α-D-glucofuranose (5d). Reaction of compound 4d (2.0 g, 5.1 mmol) with NaN3 (1.0 g, 15.4 mmol) in DMF at 115 °C afforded compound 5d. Oily liquid (1.13 g, 85% yield); IR (KBr) cm−1: 3460, 2955, 2867, 2103, 1718, 1633, 1460, 1070; 1H NMR (300 MHz, CDCl3): δ 5.89 (d, J = 3.6 Hz, 1H), 4.60 (d, J = 3.6 Hz, 1H), 4.09 (s, 2H), 3.90 (s, 1H), 3.61–3.46 (m, 5H), 2.61 (s, 1H), 1.49, 1.33 (each s, 6H); 13C NMR (75 MHz, CDCl3): δ 111.9, 105.1, 84.3, 81.3, 79.7, 68.7, 57.7, 54.4, 26.7, 26.1 ppm.
6-Azido-6-deoxy-1,2-O-isopropylidene-3-O-ethyl-α-D-glucofuranose (5e). Reaction of compound 4e (1.0 g, 2.4 mmol) with NaN3 (0.48 g, 7.2 mmol) in DMF at 115 °C afforded compound 5e. Viscous liquid (0.6 g, 90% yield); IR (KBr) cm−1: 3466, 2944, 2857, 2103, 1710, 1633, 1470, 1080; 1H NMR (300 MHz, CDCl3): δ 5.91 (d, J = 3.6 Hz, 1H), 4.57 (d, J = 3.6 Hz, 1H), 4.10–4.01 (m, 3H), 3.76–3.42 (m, 4H), 2.73 (s, 1H), 1.49, 1.32 (each s, 6H), 1.24 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 111.8, 105.0, 82.7, 81.9, 79.6, 68.8, 65.7, 54.5, 26.7, 26.1, 15.0 ppm.
Benzyl-6-azido-6-deoxy-2,3-O-isopropylidene-β-D-mannofuranose (10a). Reaction of compound 9a (1.96 g, 4.2 mmol) with NaN3 (0.82 g, 12.6 mmol) in DMF at 115 °C afforded compound 10a. Oily liquid (1.18 g, 84% yield); IR (KBr) cm−1: 3457, 3032, 2988, 2937, 2103, 1638, 1497, 1086; 1H NMR (300 MHz, CDCl3): δ 7.24 (m, 5H), 5.06–5.02 (m, 1H), 4.78 (m, 1H), 4.59–4.38 (m, 3H), 4.03–3.88 (m, 2H), 3.46–3.36 (m, 2H), 2.60 (s, 1H), 1.38, 1.24 (each s, 6H); 13C NMR (75 MHz, CDCl3): δ 137.2, 130.2, 128.5, 128.1, 112.8, 105.4, 84.8, 79.8, 79.4, 69.5, 69.1, 54.3, 25.8, 24.5 ppm.
Propyl-6-azido-6-deoxy-2,3-O-isopropylidene-β-D-mannofuranose (10b). Reaction of compound 9b (2.6 g, 6.2 mmol) with NaN3 (1.21 g, 18.7 mmol) in DMF at 115 °C afforded compound 10b. White solid (1.6 g, 90% yield); IR (KBr) cm−1: 3478, 2989, 2964, 2939, 2883, 2106, 1754, 1633, 1469, 1084; 1H NMR (300 MHz, CDCl3): δ 4.93 (s, 1H), 4.77 (dd, J = 3.9 Hz, 5.7 Hz, 1H), 4.53 (d, J = 5.7 Hz, 1H), 4.04–3.99 (m, 1H), 3.86–3.83 (m, 1H), 3.52–3.48 (m, 2H), 3.45–3.36 (m, 1H), 3.31–3.24 (m, 1H), 2.57 (s, 1H), 1.53–1.46 (m, 2H), 1.40, 1.26 (each s, 6H), 0.83 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 112.7, 106.0, 84.8, 79.8, 79.2, 69.5, 69.1, 54.3, 25.8, 24.4, 25.5, 10.4 ppm.
Isopentyl-6-azido-6-deoxy-2,3-O-isopropylidene-β-D-mannofuranose (10c). Reaction of compound 9c (2.75 g, 6.1 mmol) with NaN3 (1.2 g, 18.5 mmol) in DMF at 115 °C afforded compound 10c. White semi solid (1.53 g, 80% yield); IR (KBr) cm−1: 3453, 2922, 2871, 2101, 1744, 1633, 1465, 1088; 1H NMR (300 MHz, CDCl3): δ 4.99 (s, 1H), 4.86–4.82 (m, 1H), 4.58 (d, J = 5.7 Hz, 1H), 4.12–4.07 (m, 1H), 3.91 (dd, J = 4.2 Hz, 7.5 Hz, 1H), 3.68–3.36 (m, 4H), 1.68–1.62 (m, 1H), 1.47–1.39 (m, 5H), 1.33 (s, 3H), 0.89 (d, J = 6.6 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 112.7, 106.0, 84.8, 79.8, 79.2, 69.6, 65.8, 54.3, 38.0, 25.8, 24.8, 24.5, 22.5, 22.2 ppm.
Methyl-6-azido-6-deoxy-2,3-O-isopropylidene-β-D-mannofuranose (10d). Reaction of compound 9d (2.0 g, 5.1 mmol) with NaN3 (1.00 g, 15.4 mmol) in DMF at 115 °C afforded compound 10d. Oily liquid (1.13 g, 86% yield); IR (KBr) cm−1: 3452, 2991, 2937, 2836, 2099, 1628, 1443, 1089; 1H NMR (300 MHz, CDCl3): δ 4.90–4.82 (m, 2H), 4.58 (d, J = 5.7 Hz, 1H), 4.12–4.07 (m, 1H), 3.90 (dd, J = 3.6 Hz, 8.1 Hz, 1H), 3.60–3.44 (m, 2H), 3.31 (s, 3H), 2.62 (s, 1H), 1.47, 1.33 (each s, 6H); 13C NMR (75 MHz, CDCl3): δ 112.8, 107.0, 84.7, 79.7, 79.2, 69.6, 54.5, 54.2, 25.8, 24.5 ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) afforded the desired epoxides 12a–f.13c
hexane) afforded the desired epoxides 12a–f.13c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was treated with NaN3 and NH4Cl at 65 °C for 8 h. Upon completion of the reaction, the solvent was removed under reduced pressure, extracted with ethyl acetate and water. The organic layer was dried over anhydrous Na2SO4, filtered, concentrated under vacuum, followed by flash chromatography (ethyl acetate–hexane) to afford the desired glycosyl azido alcohols 13a–f in good yields.13c
1) was treated with NaN3 and NH4Cl at 65 °C for 8 h. Upon completion of the reaction, the solvent was removed under reduced pressure, extracted with ethyl acetate and water. The organic layer was dried over anhydrous Na2SO4, filtered, concentrated under vacuum, followed by flash chromatography (ethyl acetate–hexane) to afford the desired glycosyl azido alcohols 13a–f in good yields.13c6-(3-O-Benzyl-1,2-O-isopropylidene-α-D-glucofuranose)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 6a. Compound 5a (100 mg, 0.3 mmol), sodium hydride (21 mg, 0.9 mmol) and propargyl bromide (0.034 ml, 4 mmol) were reacted in DMF (8 ml) using the procedure described above to give 6a (102 mg, 92%) as a white solid; Rf = 0.40 (60% ethyl acetate–n-hexane); MS: m/z 374 [M + H]+; IR (KBr) cm−1 2963, 2927, 2856, 1628, 1455, 1375, 1261, 1094, 1023, 801: 1H NMR (300 MHz, CDCl3): δ 7.39 (s, 1H), 7.26–7.24 (m, 5H), 5.87 (d, J = 3.6 Hz, 1H), 4.92 (d, J = 15.0 Hz, 1H), 4.68–4.46 (m, 5H), 4.19–4.08 (m, 4H), 1.43, 1.26 (each s, 6H); 13C NMR (75 MHz, CDCl3): δ 137.1, 130.2, 128.5, 128.1, 127.7, 112.0, 105.1, 81.8, 81.0, 80.2, 72.2, 70.3, 61.7, 48.1, 26.5, 25.9 ppm.
6-(1,2-O-Isopropylidene-3-O-propyl-α-D-glucofuranose)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 6b. 5b (150 mg, 0.52 mmol), sodium hydride (37 mg, 1.5 mmol) and propargyl bromide (0.06 ml, 0.6 mmol) were reacted in DMF (8 ml) using the procedure described above to give 6b (162 mg, 96%) as a yellowish viscous liquid; Rf = 0.25 (40% ethyl acetate–n-hexane); MS: m/z 326 [M + H]+; IR (KBr) cm−1 3142, 2965, 2936, 1636, 1455, 1375, 1217, 1081, 1022: 1H NMR (300 MHz, CDCl3): δ 7.48 (s, 1H), 5.91 (d, J = 3.3 Hz, 1H), 5.09 (d, J = 14.7 Hz, 1H), 4.82–4.59 (m, 3H), 4.25–4.21 (m, 3H), 3.99 (d, J = 2.1 Hz, 1H), 3.64 (dd, J = 6.3 Hz, 15.3 Hz, 1H), 3.45 (dd, J = 6.6 Hz, 15.3 Hz, 1H), 1.64–1.57 (m, 2H), 1.50, 1.33 (each s, 6H), 0.93 (t, J = 7.5 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 130.1, 127.7, 111.8, 105.0, 81.8, 81.5, 80.2, 72.0, 70.3, 61.6, 48.0, 26.5, 25.9, 22.6, 10.3 ppm.
6-(3-O-Isopentyl-1,2-O-isopropylidene-α-D-glucofuranose)-6,7-dihydro-4H [1,2,3]triazolo[5,1-c][1,4]oxazine, 6c. 5c (150 mg, 0.47 mmol), sodium hydride (34 mg, 1.42 mmol) and propargyl bromide (0.054 ml, 0.61 mmol) were reacted in DMF (8 ml) using the procedure described above to give 6c (146 mg, 88%) as a white solid; Rf = 0.25 (40% ethyl acetate–n-hexane); MS: m/z 354 [M + H]+; IR (KBr) cm−1 3429, 2963, 2924, 2936, 1622, 1453, 1374, 1217, 1126, 1082, 1020: 1H NMR (300 MHz, CDCl3): δ 7.39 (s, 1H), 5.82 (d, J = 3.6 Hz, 1H), 5.2–4.97 (m, 1H), 4.71–4.49 (m, 3H), 4.15–4.10 (m, 3H), 3.89 (d, J = 2.7 Hz, 1H), 3.62–3.57 (m, 1H), 3.44–3.37 (m, 1H), 1.61–1.53 (m, 1H), 1.41–1.37 (m, 5H), 1.24 (s, 3H), 0.81 (d, J = 6.3 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 130.1, 127.8, 111.8, 105.1, 81.7, 81.6, 80.2, 70.3, 68.7, 61.6, 48.0, 38.2, 26.5, 25.9, 24.6, 22.3, 22.0 ppm.
6-(1,2-O-Isopropylidene-3-O-methyl-α-D-glucofuranose)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 6d. 5d (130 mg, 0.5 mmol), sodium hydride (0.36 mg, 1.5 mmol) and propargyl bromide (0.057 ml, 0.65 mmol) were reacted in DMF (8 ml) using the procedure described above to give 6d (133 mg, 90%) as a yellowish viscous liquid; Rf = 0.25 (40% ethyl acetate–n-hexane); MS: m/z 298 [M + H]+; IR (KBr) cm−1 3442, 2988, 2935, 1635, 1455, 1375, 1217, 1082: 1H NMR (300 MHz, CDCl3): δ 7.39 (s, 1H), 5.82 (d, J = 3.9 Hz, 1H), 5.01 (d, J = 15.0 Hz, 1H), 4.75 (d, J = 15.0 Hz, 1H), 4.62–4.53 (m, 2H), 4.19–4.04 (m, 3H), 3.82 (d, J = 2.7 Hz, 1H), 3.38 (s, 3H), 1.42, 1.26 (each s, 6H); 13C NMR (75 MHz, CDCl3): 130.1, 127.7, 111.8, 105.0, 83.2, 81.2, 80.0, 70.3, 61.6, 57.9, 47.8, 26.5, 25.8 ppm.
6-(3-O-Ethyl-1,2-O-isopropylidene-α-D-glucofuranose)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 6e. Compound 5e (100 mg, 0.36 mmol), sodium hydride (26 mg, 1.09 mmol) and propargyl bromide (0.041 ml, 0.46 mmol) were reacted in DMF (8 ml) using the procedure described above to give 6e (97 mg, 87%) as a viscous liquid; Rf = 0.25 (40% ethyl acetate–n-hexane); MS: m/z 312 [M + H]+; IR (KBr) cm−1 2976, 2930, 2886, 1738, 1551, 1449, 1378, 1088, 888, 474: 1H NMR (300 MHz, CDCl3): δ 7.49 (s, 1H), 5.94–5.92 (m, 1H), 5.09 (dd, J = 2.1 Hz, 15.0 Hz, 1H), 4.83–4.59 (m, 3H), 4.30–4.17 (m, 3H), 4.01 (s, 1H), 3.74 (t, J = 7.2 Hz, 1H), 3.58–3.53 (m, 1H), 1.51, 1.34 (each s, 6H), 1.22 (t, J = 6.9 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 130.2, 127.9, 111.9, 105.1, 82.1, 81.4, 80.1, 70.6, 66.0, 61.8, 48.1, 26.6, 25.9, 15.0 ppm.
6-(Benzyl-1,2-O-isopropylidene-3-O-β-D-mannofuranose)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 11a. Compound 10a (130 mg, 0.38 mmol), sodium hydride (27 mg, 1.16 mmol) and propargyl bromide (0.043 ml, 0.5 mmol) were reacted in DMF (8 ml) using procedure described above to give 11a (119 mg, 84%) as Rf = 0.25 (35% ethyl acetate–n-hexane); MS: m/z 374 [M + H]+; white solid; IR (KBr) cm−1 3421, 3090, 2988, 2941, 2913, 1743, 1682, 1447, 1380, 1255, 1106, 1083: 1H NMR (300 MHz, CDCl3): δ 7.40 (s, 1H), 7.29–7.24 (m, 5H), 5.05–4.96 (m, 2H), 4.78–4.73 (m, 2H), 4.60–4.43 (m, 4H), 4.16–3.96 (m, 3H), 1.37, 1.24 (each s, 6H); 13C NMR (75 MHz, CDCl3): δ 137.1, 130.3, 128.3, 127.8, 127.8, 112.6, 105.6, 84.5, 79.6, 79.1, 70.7, 69.3, 61.7, 47.6, 25.7, 24.4 ppm.
6-(Propyl-1,2-O-isopropylidene-3-O-β-D-mannofuranose)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 11b. Compound 10b (150 mg, 0.52 mmol), sodium hydride (37 mg, 1.5 mmol) and propargyl bromide (0.060 ml, 0.67 mmol) were reacted in DMF (8 ml) using the procedure described above to give 11b (158 mg, 94%) as a yellowish viscous liquid; Rf = 0.25 (35% ethyl acetate–n-hexane); MS: m/z 326 [M + H]+; IR (KBr) cm−1 3434, 3137, 2963, 2938, 2878, 1714, 1630, 1456, 1374, 1210, 1163, 1043, 862: 1H NMR (300 MHz, CDCl3): δ 7.41 (s, 1H), 5.05–4.95 (m, 2H), 4.82–4.77 (m, 2H), 4.70–4.60 (m, 1H), 4.54 (d, J = 5.7 Hz, 1H), 4.18–4.15 (m, 2H), 4.00 (d, J = 3.9 Hz, 1H), 3.51 (dd, J = 6.9 Hz, 13.8 Hz, 1H), 3.31 (d, J = 6.6 Hz, 15.9 Hz, 1H), 1.53–1.46 (m, 2H), 1.38, 1.25 (each s, 6H), 0.83 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 130.3, 127.7, 112.5, 106.0, 84.6, 79.4, 79.1, 70.8, 69.0, 61.6, 47.6, 25.7, 24.3, 22.3, 10.2 ppm.
6-(Isopentyl-1,2-O-isopropylidene-3-O-β-D-mannofuranose)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 11c. Compound 10c (130 mg, 0.41 mmol), sodium hydride (29 mg, 1.2 mmol) and propargyl bromide (0.047 ml, 5.3 mmol) were reacted in DMF (8 ml) using the procedure described above to give 11c (133 mg, 92%) as a yellowish viscous liquid; Rf = 0.25 (35% ethyl acetate–n-hexane); MS: m/z 354 [M + H]+; IR (KBr) cm−1 3444, 3130, 2983, 2948, 2870, 1710, 1604, 1456, 1374, 1211, 1150, 1045, 860: 1H NMR (300 MHz, CDCl3): δ 7.43 (s, 1H), 5.05–4.53 (m, 6H), 4.17–3.98 (m, 3H), 3.63–3.56 (m, 1H), 3.37 (dd, J = 6.6 Hz, 15.9 Hz, 1H), 1.64–1.53 (m, 1H), 1.39, 1.27 (each s, 6H), 0.83 (d, J = 6.6 Hz, 6H); 13C NMR (75 MHz, CDCl3): 130.4, 127.9, 112.7, 106.2, 74.7, 79.5, 79.1, 71.0, 65.9, 61.8, 47.8, 38.0, 25.8, 24.7, 24.4, 22.4, 22.1 ppm.
6-(Methyl-1,2-O-isopropylidene-3-O-β-D-mannofuranose)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 11d. Compound 10d (120 mg, 0.46 mmol), NaH (33 mg, 1.3 mmol) and propargyl bromide (0.053 ml, 5.8 mmol) were reacted in DMF (8 ml) using the procedure described above to give 11d (117 mg, 86%) as a white solid; Rf = 0.25 (35% ethyl acetate–n-hexane); MS: m/z 298 [M + H]+; IR (KBr) cm−1 3137, 2989, 2947, 2833, 1716, 1625, 1450, 1381, 1270, 1207, 1160, 1105, 1049, 860: 1H NMR (300 MHz, CDCl3): δ 7.50 (s, 1H), 5.10 (dd, J = 15.9 Hz, 1H), 4.93–4.85 (m, 3H), 4.75 (d, J = 8.1 Hz, 1H), 4.60 (d, J = 5.7 Hz, 1H), 4.27–4.25 (m, 2H), 4.06 (t, J = 3.0 Hz, 1H), 3.34 (s, 3H), 1.46, 1.34 (each s, 6H); 13C NMR (75 MHz, CDCl3): δ 130.4, 127.9, 112.8, 107.1, 84.5, 79.5, 79.1, 70.9, 61.8, 54.6, 47.7, 25.8, 24.4.
6-(1,2:5,6-Di-O-isopropylidene-3-O-oxymethylene-α-D-glucofuranose)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 14a. Compound 13a (166 mg, 0.46 mmol), sodium hydride (33 mg, 1.3 mmol) and propargyl bromide (0.053 ml, 0.59 mmol) were reacted in DMF (8 ml) using the procedure described above to give 14a (158 mg, 87%) as a yellowish viscous liquid; Rf = 0.30 (60% ethyl acetate–n-hexane); MS: m/z 398 [M + H]+; IR (KBr) cm−1 3447, 2987, 2935, 1713, 1634, 1456, 1374, 1216, 1073, 1020: 1H NMR (300 MHz, CDCl3): δ 7.42 (s, 1H), 5.82 (s, 1H), 5.04 (d, J = 15.0 Hz, 1H), 4.79–4.74 (m, 1H), 4.51 (m, 2H), 4.20–3.75 (m, 9H), 1.43, 1.34, 1.32, 1.25 (each s, 12H); 13C NMR (75 MHz, CDCl3): δ 130.3, 127.9, 111.9, 109.1, 105.2, 83.2, 82.5, 81.1, 72.7, 72.1, 70.4, 67.5, 61.8, 47.0, 26.6, 26.6, 26.0, 25.1 ppm.
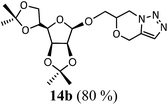
6-(2,3:4,6-Di-O-isopropylidene-1-O-oxymethylene-β-D-mannofuranose)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 14b. Compound 13b (140 mg, 0.39 mmol), sodium hydride (28 mg, 1.1 mmol) and propargyl bromide (0.045 ml, 0.5 mmol) were reacted in DMF (8 ml) using the procedure described above to give 14b (123 g, 80%) as a brownish solid; Rf = 0.30 (60% ethyl acetate–n-hexane); MS: m/z 398 [M + H]+; IR (KBr) cm−1 3456, 3140, 2995, 2946, 1644, 1466, 1375, 1210, 1078: 1H NMR (300 MHz, CDCl3): δ 7.49 (s, 1H), 5.14–5.04 (m, 2H), 4.78 (m, 2H), 4.64 (s, 1H), 4.49–4.41 (m, 2H), 4.13–3.85 (m, 2H), 3.72 (m, 1H), 1.45, 1.43, 1.37, 1.32 (each s, 12H); 13C NMR (75 MHz, CDCl3): δ 130.1, 127.8, 112.6, 108.9, 106.6, 84.7, 80.3, 79.2, 72.8, 72.4, 72.2, 66.9, 66.5, 61.7, 46.7, 26.6, 25.6, 24.9, 24.3 ppm.
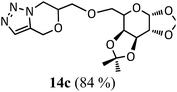
6-(1,2:3,4-Di-O-isopropylidene-6-O-oxymethylene-α-D-galactopyranose)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 14c. Compound 13c (140 mg, 0.39 mmol), sodium hydride (28 mg, 1.1 mmol) and propargyl bromide (0.045 ml, 0.5 mmol) were reacted in DMF (8 ml) using the procedure described above to give 14c (130 g, 84%) as a yellowish viscous liquid; Rf = 0.30 (60% ethyl acetate–n-hexane); MS: m/z 398 [M + H]+; IR (KBr) cm−1 3457, 3142, 2988, 2936, 1640, 1374, 1217, 1078: 1H NMR (300 MHz, CDCl3): δ 7.49 (s, 1H), 5.53 (d, J = 4.5 Hz, 1H), 5.11 (d, J = 15.0 Hz, 1H), 4.83 (d, J = 15.0 Hz, 1H), 4.62–4.55 (m, 2H), 4.33–4.16 (m, 3H), 4.09–4.00 (m, 1H), 3.90–3.70 (m, 4H), 1.54, 1, 1.45, 1.33 (each s, 12H); 13C NMR (75 MHz, CDCl3): δ 130.4, 128.0, 109.3, 108.6, 96.2, 72.7, 71.1, 70.7, 70.6, 70.4, 67.1, 66.9, 61.8, 47.2, 25.9, 25.8, 24.7, 24.2 ppm.
6-(Methyl-2,3-O-isopropylidene-5-O-oxymethylene-β-D-ribofuranose)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 14d. Compound 13d (100 mg, 0.33 mmol), sodium hydride (23 mg, 1.0 mmol) and propargyl bromide (0.038 ml, 0.42 mmol) were reacted in DMF (8 ml) using the procedure described above to give 14d (95 mg, 85%) as a yellowish viscous liquid; Rf = 0.30 (60% ethyl acetate–n-hexane); MS: m/z 342 [M + H]+; IR (KBr) cm−1 2925, 2855, 1724, 1635, 1455, 1383, 1216, 1165, 1075, 1020, 857: 1H NMR (300 MHz, CDCl3): δ 7.48 (s, 1H), 5.11 (d, J = 15.3 Hz, 1H), 4.96 (s, 1H), 4.84 (d, J = 15.0 Hz, 1H), 4.67–4.53 (m, 3H), 4.36–4.05 (m, 3H), 3.85–3.70 (m, 2H), 3.58–3.56 (m, 2H), 3.32 (s, 3H), 1.48, 1.32 (each s, 6H); 13C NMR (75 MHz, CDCl3): δ 130.3, 127.8, 112.2, 109.1, 84.8, 84.6, 81.7, 72.5, 72.4, 70.8, 61.7, 54.6, 46.9, 26.1, 24.6 ppm.
6-(3-O-Benzyl-1,2-O-isopropylidene-5-O-oxymethylene-α-D-galactopyranose)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 14e. Compound 13e (100 mg, 0.26 mmol), sodium hydride (19 mg, 0.79 mmol) and propargyl bromide (0.03 ml, 0.33 mmol) were reacted in DMF (8 ml) using the procedure described above to give 14e (95 mg, 88%) as a yellowish liquid; Rf = 0.30 (60% ethyl acetate–n-hexane); MS: m/z 418 [M + H]+; IR (KBr) cm−1 3409, 2987, 2938, 1712, 1454, 1373, 1138, 869: 1H NMR (300 MHz, CDCl3): δ 7.48 (s, 1H), 7.31–7.27 (m, 5H), 5.94 (d, J = 3.6 Hz, 1H), 5.08 (dd, J = 1.8 Hz, 15.0 Hz, 1H), 4.82–4.38 (m, 7H), 4.18–3.65 (m, 6H), 1.49, 1.32 (each s, 6H); 13C NMR (75 MHz, CDCl3): δ 137.3, 130.4, 128.5, 128.0, 127.6, 111.7, 105.1, 82.0, 81.7, 79.1, 72.7, 72.6, 71.8, 71.3, 69.4, 61.8, 46.9, 26.6, 26.1 ppm.
6-(Morpholine)-6,7-dihydro-4H-[1,2,3]triazolo[5,1-c][1,4]oxazine, 14f. Compound 13f (80 mg, 0.43 mmol), sodium hydride (30 mg, 1.2 mmol) and propargyl bromide (0.049 ml, 0.55 mmol) were reacted in DMF (8 ml) using the procedure described above to give 14f (77 mg, 80%) as a reddish semi solid; IR (KBr) cm−1 Rf = 0.15 (70% ethyl acetate–n-hexane); MS: m/z 225 [M + H]+; 3416, 2980, 2934, 1710, 1630, 1456, 1116, 1058, 868, 471: 1H NMR (300 MHz, CDCl3): δ 7.49 (s, 1H), 5.10 (d, J = 15.0 Hz, 1H), 4.81 (d, J = 15.0 Hz, 1H), 4.56 (d, J = 10.2 Hz, 1H), 4.14–4.07 (m, 2H), 3.75–3.72 (m, 4H), 2.84–2.75 (m, 1H), 2.64–2.57 (m, 4H); 13C NMR (75 MHz, CDCl3): δ 130.4, 127.0, 71.8, 66.7, 61.8, 60.4, 54.3, 48.4 ppm.
Acknowledgements
VKT gratefully acknowledges Council of Scientific & Industrial Research (CSIR), New Delhi (Grant No. 02(0173)/13/EMR-II) for the funding and CISC, Department of Chemistry, Banaras Hindu University for providing spectroscopic data of synthesized compounds. KBM gratefully acknowledges UGC, New Delhi, for a fellowship (JRF & SRF).References
- (a) R. Dua, S. Shrivastava, S. K. Sonwane and S. K. Srivastava, Adv. Biol. Res., 2011, 5, 120 CAS; (b) A. F. Pozharskii, A. Soldatenkov and A. R. Katritzky, Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry and Applications, 2011 Search PubMed; (c) B. B. Mishra, D. Kumar, A. Mishra and V. K. Tiwari, Adv. Heterocycl. Chem., 2012, 107, 41 CrossRef CAS PubMed; (d) R. K. Arigela, A. K. Mandadapu, S. K. Sharma, B. Kumar and B. Kundu, Org. Lett., 2012, 14, 1804 CrossRef CAS PubMed.
- (a) K. B. Mishra and V. K. Tiwari, J. Org. Chem., 2014, 79, 5752 CrossRef CAS PubMed; (b) Y. S. Reddy, A. P. John Pal, P. Gupta, A. A. Ansari and Y. D. Vankar, J. Org. Chem., 2011, 76, 5972 CrossRef CAS PubMed; (c) B. Whittaker, C. Steele, D. Hardick, M. Dale, V. Pomel, A. Quattropani and D. Beher, Eur. Pat. Appl., EP 2687528 A1 20140122, 2014; (d) K. B. Mishra, B. B. Mishra and V. K. Tiwari, Carbohydr. Res., 2014, 399, 2 CrossRef CAS PubMed.
- For reviews, see: (a) R. Wijtmans, M. K. S. Vink, H. E. Schoemaker, F. L. van Delft, R. H. Blaauw and F. P. J. T. Rutjes, Synthesis, 2004, 641 CAS; (b) K. Audouze, E. O. Nielsen and D. Peters, J. Med. Chem., 2004, 47, 3089 CrossRef CAS PubMed; (c) T. V. Abramova, P. A. Bakharev, V. Svetlana and V. N. Silinikov, Tetrahedron Lett., 2004, 45, 4361 CrossRef CAS PubMed; (d) M. Hajos, J. C. Fleishaker, J. K. Filipiak-Reisner, M. T. Brown and E. H. F. Wong, CNS Drug Rev., 2004, 10, 23 CrossRef CAS PubMed and references therein.
- J. W. W. Chang and P. W. H. Chan, Angew. Chem., Int. Ed., 2008, 47, 1138 CrossRef CAS PubMed.
- (a) G. C. Tron, T. Pirali, R. A. Billington, P. L. Canonico, G. Sorba and A. A. Genazzani, Med. Res. Rev., 2008, 28, 278 CrossRef CAS PubMed; (b) P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905 CrossRef CAS PubMed; (c) D. Kushwaha, P. Dwivedi, S. K. Kuanar and V. K. Tiwari, Curr. Org. Synth., 2013, 10, 90 CrossRef CAS.
- (a) F. C. Da Silva, M. C. B. V. de Souza, I. I. P. Frugulhetti, H. C. Castro, S. L. O. Souza, T. M. L. de Souza, D. Q. Rodrigues, A. M. T. Souza, P. A. Abreu, F. Passamani, C. R. Rodrigues and V. F. Ferreira, Eur. J. Med. Chem., 2009, 44, 373 CrossRef CAS PubMed; (b) R. Alvarez, S. Velazquez, A. San-Felix, S. Aquaro, E. de Clercq, C. F. Perno, A. Karlsson, J. Balzarini and M. J. Camarasa, J. Med. Chem., 1994, 37, 4185 CrossRef CAS.
- L. S. Kallander, Q. Lu, W. Chen, T. Tomaszek, G. Yang, D. Tew, T. D. Meek, G. A. Hofmann, C. K. Schulz-Pritchard, W. W. Smith, C. A. Janson, M. D. Ryan, G. F. Zhang, K. O. Johanson, R. B. Kirkpatrick, T. F. Ho, P. W. Fisher, M. R. Mattern, R. K. Johnson, M. J. Hansbury, J. D. Winkler, K. W. Ward, D. F. Veber and S. K. Thompson, J. Med. Chem., 2005, 48, 5644 CrossRef CAS PubMed.
- (a) T. D. Heightman, A. Vasella, K. E. Tsitsanou, S. E. Zographos, V. T. Skamnaki and N. G. Oikonomakos, Helv. Chim. Acta, 1998, 81, 853 CrossRef CAS PubMed; (b) T. M. Krulle, C. D. Fuente, L. Pickering, R. T. Aplin, K. E. Tsitsanou, S. E. Zographos, N. G. Oikonomakos, R. J. Nash, R. C. Griffiths and G. W. Fleet, Tetrahedron: Asymmetry, 1997, 8, 3807 CrossRef CAS; (c) T. D. Heightman, M. Locatelli and A. Vasella, Helv. Chim. Acta, 1996, 79, 2190 CrossRef CAS PubMed.
- (a) For general reviews on chemistry and applications of 1,2,3-triazoles: W.-Q. Fan and A. R. Katritzky, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Elsevier Science, Oxford, U.K., 1996, vol. 4, pp. 11–126 Search PubMed; (b) N. H. Morgan, Eur. Pat. Appl. EP 437979 A2 19910742, 1991.
- F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210 CrossRef CAS PubMed.
- For selected examples, see: (a) P. L. Golas and K. Matyjaszewski, Chem. Rev., 2010, 39, 1338 RSC; (b) J. E. Hein and V. V. Fokin, Chem. Soc. Rev., 2010, 39, 1302 RSC; (c) L. Liang and D. Astruc, Chem. Rev., 2011, 255, 2933 CAS.
- (a) L. Zhang, X. Chen, P. Xue, H. H. Y. Sun, I. D. Williams, K. B. Sharpless, V. V. Fokin and G. Jia, J. Am. Chem. Soc., 2005, 127, 15998 CrossRef CAS PubMed; (b) A. Tam, U. Arnold, M. B. Soellner and R. T. Raines, J. Am. Chem. Soc., 2007, 129, 12670 CrossRef CAS PubMed; (c) M. M. Majireck and S. M. Weinreb, J. Org. Chem., 2006, 71, 8680 CrossRef CAS PubMed; (d) L. K. Rasmussen, B. C. Boren and V. V. Fokin, Org. Lett., 2007, 9, 5337 CrossRef CAS PubMed; (e) B. C. Boren, S. Narayan, L. K. Rasmussen, L. Zhang, H. Zhao, Z. Lin, G. Jia and V. V. Fokin, J. Am. Chem. Soc., 2008, 130, 8923 CrossRef CAS PubMed; (f) J. R. Johansson, P. Lincoln, B. Norden and N. Kann, J. Org. Chem., 2011, 76, 2355 CrossRef CAS PubMed; (g) D. Wang, L. Salmon, J. Ruiz and D. Astruc, Chem. Commun., 2013, 49, 6956 RSC.
- (a) D. Kumar, A. Mishra, B. B. Mishra, S. Bhattacharya and V. K. Tiwari, J. Org. Chem., 2013, 78, 899 CrossRef CAS PubMed; (b) D. Kumar, K. B. Mishra, B. B. Mishra, S. Mondal and V. K. Tiwari, Steroids, 2014, 80, 71 CrossRef CAS PubMed; (c) K. B. Mishra, R. C. Mishra and V. K. Tiwari, RSC Adv., 2015, 5, 51779 RSC.
- M. Baumann and I. R. Baxendale, Beilstein J. Org. Chem., 2013, 9, 2265 CrossRef PubMed.
- (a) B. Lewandowski and S. Jarosz, Org. Lett., 2010, 12, 2532 CrossRef CAS PubMed; (b) N. D. Adhikary and P. Chattopadhyay, J. Org. Chem., 2012, 77, 5399 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR for all the novel compounds has been provided. See DOI: 10.1039/c5ra17181d |
| This journal is © The Royal Society of Chemistry 2015 |