A highly selective colorimetric Cys sensor based on core-substituted naphthalene diimides†
Sharad R. Bobea,
Rajesh S. Bhosale*a,
Santosh P. Goskulwada,
Avinash L. Puyadc,
Sheshanath V. Bhosale*b and
Sidhanath V. Bhosale*a
aPolymers and Functional Material Division, CSIR-Indian Institute of Chemical Technology, Hyderabad 500607, Telangana, India. E-mail: bhosale@iict.res.in; bhosale.iict@gov.in; bhosaleo4@gmail.com
bSchool of Applied Sciences, RMIT University, GPO Box 2476V, Melbourne 3001, Victoria, Australia. E-mail: sheshanath.bhosale@rmit.edu.au; Tel: +61 399252680
cSchool of Chemical Sciences, Swami Ramanand Teerth Marathwada University, Nanded-431 606, Maharashtra, India
First published on 11th November 2015
Abstract
This study presents the use of a core-substituted naphthalene diimide (NDI) probe for the selective sensing of cysteine (Cys). The UV-vis and colorimetric study showed that the NDI probe is highly selective towards Cys detection without any interference from other amino acids. We have also found that in the presence of Cys, the naked-eye color of receptor 1 changed from yellow to colourless. The sensing mechanism for Cys detection is based on the photoinduced electron transfer (PET) effect.
Cysteine (Cys) is one of the important amino acids amongst the natural amino acids and plays a crucial role in living systems.1 The deficiency of Cys is responsible for many diseases such as the depigmentation of hair, reduced growth in childhood, liver damage, edema, lethargy, loss of fat and muscles, skin lesions, cancer and weakness.2 In addition, Cys can also induce hypoglycemic brain damage.3 Therefore, the detection and monitoring of Cys is very important from the human health care stand point. Several strategies have been developed to detect Cys such as high-performance liquid chromatography (HPLC), high performance capillary electrophoresis (HPCE), mass spectrometry, FTIR detection and electrochemical detection methods.4 There are some methods based on fluorescence spectral techniques that have also been reported for the detection of Cys.5 However, these reported methods require sophisticated instruments and time consuming experimental procedures. Therefore, researchers have focused on the development of more attractive approaches based on colorimetric detection methods that can improve the simplicity, selectivity and sensitivity of Cys detection in buffered media. A literature survey revealed that the selective detection of Cys based on sensing color changes are limited in number.6 Therefore, a colorimetric method used for the selective detection of Cys can be established.
Recently, naphthalene diimide (NDI) derivatives have received increasing amounts of attention because of their applications in materials and biological science.7 However, the NDI structure and planar π-electron deficient aromatic nature exhibits different electronic and optical properties based on core- or imide-substitution. This makes NDI derivatives ideal candidates to investigate for sensing applications.8 However, easily synthesised NDI probes for Cys sensing remain unexplored.
Herein, we report a colorimetric assay for Cys, which was based on the competitive binding of Cys with a NDI conjugate that bears formyl functionality at the core separated by a thiophene spacer. To the best of our knowledge, this is the first report describing an optically active NDI derivative used for sensing Cys over other amino acids. NDI can form a thiazolidine with Cys through a formyl-Cys reaction and results in a significant color change. The thiazolidine formation can be monitored easily using 1H-NMR spectroscopy of the NDI receptor.
The NDI receptor 1 was synthesized (Scheme 1) according to the previously reported method.9 The synthesised compound 1 was fully characterized by means of FTIR, 1H-NMR, 13C-NMR and mass spectroscopy (Fig. S1–S4†).
At first glance the naked-eye detection experiment was performed in DMSO upon the addition of the various amino acids, including Asp, His, Ile, Glu, Pro, Gln, Thr, Lys, Asn, Phe, Ser, Val, Trp, Tyr, Cys and HCys (125 equiv., H2O; pH 7.4 buffered with 0.1 M HEPES), to a solution of the receptor 1 (1 × 10−4 M). When Cys was added to the receptor 1 solution, the color of the resulting solution changed from yellow to colourless (Fig. 1), whereas the addition of other amino acids, such as Asp, His, Ile, Glu, Pro, Gln, Thr, Lys, Asn, Phe, Ser, Val, Trp, Tyr and HCys, exhibits no color change under the same conditions. We also examined the time dependent naked-eye color change of 1 upon the addition of various amino acids for 0–24 h (Fig. S5†). This clearly indicated that receptor 1 selectively recognised Cys.
 | ||
| Fig. 1 The color changes of the receptor 1 (1 × 10−4 M) in DMSO in the presence of various amino acids (125 equiv., H2O; pH 7.4; buffered with 0.1 M HEPES). | ||
Furthermore, we investigated the practical application of probe 1 for the naked-eye detection of Cys at various concentrations (0–100 equiv.) along with increasing time (0–48 h) (Fig. S6†). It was clearly observed that the color of probe 1 does not change over 48 h for Cys (0–25 equiv.), whereas upon increasing the concentration of Cys, for example from 50 to 100 equivalents, the color of probe 1 changes from yellow to colorless (0–48 h).
Furthermore, the UV-vis absorption spectrum of receptor 1 was studied in DMSO. The solution of receptor 1 showed four absorption bands at 302 nm, 364 nm, 386 nm and 434 nm. Upon the addition of various amino acids, viz., Asp, His, Ile, Glu, Pro, Gln, Thr, Lys, Asn, Phe, Ser, Val, Trp, Tyr and HCys (H2O; pH 7.4; buffered with 0.1 M HEPES), to a solution of receptor 1, slight changes in its absorption spectrum were observed. In the presence of Cys a significant absorption change was observed (Fig. 2a). It can be observed from Fig. S7† that upon the addition of increasing amounts of Cys, the absorption bands of NDI at 302 nm showed a blue shift towards 290 nm and the bands at 364 nm to 367 nm, 386 nm to 399 nm and 434 nm to 459 nm were red shifted with a gradual decrease in their intensity, which was attributed to the structural change that developed in receptor 1, i.e. the formation of thiazolidine ring 3 (Scheme 2). The binding stoichiometry between the receptor 1 and Cys was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, obtained from the Job's plot (Fig. S8†).
2, obtained from the Job's plot (Fig. S8†).
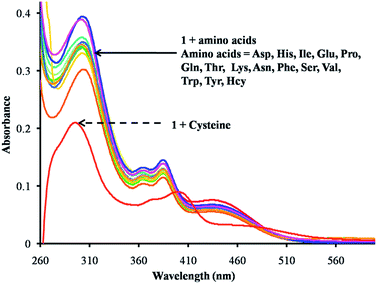 | ||
| Fig. 2 UV-vis spectra of the receptor 1 (1 × 10−5 M) upon the addition of various amino acids (125 equiv.) in H2O (pH 7.4; buffered with 0.1 M HEPES). | ||
The change in the structural motif, i.e., the formation of thiazolidine derivative 3 (Scheme 2), was also confirmed using 1H-NMR spectroscopy. Upon the addition of Cys (125 equiv.) to the receptor 1, the 1H-NMR signal for the aldehyde at 10.05 ppm disappeared and a new peak appeared at 4.9 ppm, which can be assigned to the thiazolidine methine protons (Fig. 3). These results indicated that upon the addition of Cys to receptor 1, the interaction of aldehyde with Cys resulted in the formation of a thiazolidine heterocycle (Scheme 2).
 | ||
| Fig. 3 1H NMR spectral changes of receptor 1 (in DMSO-d6) in the absence (a) and presence (b) of 125 equivalents Cys. | ||
To get a detailed insight for the selectivity of receptor 1 towards amino acids, we examined the fluorescence spectroscopy changes upon the addition of 125 equivalents of various amino acids (Asp, His, Ile, Glu, Pro, Gln, Thr, Lys, Asn, Phe, Ser, Val, Trp, Tyr, Cys and HCys) in water (0.1 M HEPES for pH 7.4). Upon excitation at 375 nm, receptor 1 displayed fluorescence emission bands at 412 nm and 430 nm. These emission bands at 412 and 430 nm underwent fluorescence quenching selectively upon the addition of an increasing concentration of Cys (Fig. 4). When compared to Cys, the other amino acids studied show negligible changes (Fig. S9†). These fluorescence emission experiments indicated that receptor 1 displayed a high selectivity towards Cys.
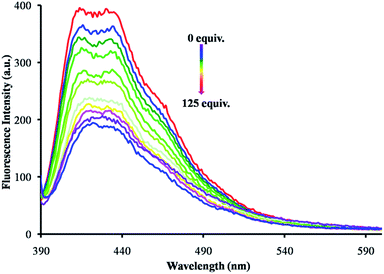 | ||
| Fig. 4 Emission spectra of receptor 1 (1 × 10−5 M, λex = 375 nm) in DMSO upon the addition of an increasing concentration of Cys (H2O; buffered with 0.1 M HEPES for pH 7.4). | ||
On the basis of the 1H-NMR and fluorescence emission data obtained, we can presume that the emission bands of receptor 1 were quenched upon the addition of Cys and indicated the formation of a five-membered thiazolidine ring possessing a lone pair of electrons at the nitrogen atom. These lone pair of electrons quench the fluorescence of NDI via a PET mechanism.
To get an insight for the dependence of the CHO group on the optical properties of 1 and 3, calculations were performed based on density functional theory (DFT) employing the B3LYP/6-31G** level of theory using the Gaussian 09 ab initio suite/DFT quantum chemical simulation package.10 At the same level, the frequencies were calculated to ensure that each of the optimized geometries have all real (positive) frequencies. The frontier molecular orbitals of both 1 and 3 were calculated and their pictures were generated using Avogadro (Fig. 5).11,12 Fig. 5 shows the molecular orbital plots (HOMO and LUMO) of the NDI probe (1 and 3). In the HOMO (−6.71 eV) of 1, the electron density mainly resides on thiophene ring and carbonyl group (Fig. 5), whereas in the LUMO (−3.74 eV) the electron density resides on NDI core. The energy difference between the HOMO and LUMO of 1 is 2.97 eV. The addition of cysteine to 1 results in 3 bearing a thiazolidine ring system. In HOMO (−5.98 eV) of 3, electron density mainly resides on the thiophene ring and thiazolidine ring, and LUMO (−3.34 eV) is mainly located on the NDI core. The energy gap between the HOMO and LUMO of 3 is 2.64 eV. In this state, a PET from the thiazolidine ring system to the NDI core is significant. The fluorescence emission intensity of the compound 3 was significantly decreased (Fig. 4).
 | ||
| Fig. 5 The frontier molecular orbitals of 1 and 3 calculated using the DFT method at the B3LYP/6-31G** level of theory. | ||
For the practical application of receptor 1 as a Cys sensor, high selectivity is necessary. At first, UV-vis absorption spectroscopy was employed to investigate the competitive sensing of Cys using receptor 1. The competitive experiments were carried out upon the addition of Cys (125 equiv.) to a solution of receptor 1 in the presence of other amino acids, viz., Asp, His, Ile, Glu, Pro, Gln, Thr, Lys, Asn, Phe, Ser, Val, Trp, Tyr, Cys and HCys in water (buffered with 0.1 M HEPES for pH 7.4) (125 equiv.). However, no significant change in the UV-vis absorption spectral profile of 1 was observed in the presence of the other amino acids studied in comparison to that obtained by the addition of only Cys to receptor 1 (Fig. 6a). Furthermore, colorimetric competitive experiments were also performed to explore the ability of 1 as a selective colorimetric sensor for Cys in the presence of various amino acids. The presence of other amino acids had no influence on the color change (Fig. 6b). Thus, receptor 1 shows selectivity towards Cys over the other amino acids studied.
We found that under the same conditions, receptor 1 did not react with HCys, indicating that the presence of thiophene ring in between the electron deficient naphthalene diimide and aldehyde can decrease the electropositivity of the aldehyde group and reduce the reactivity. Thus, the presence of the thiophene ring system in between NDI and the aldehyde groups plays an important role in the receptor selectivity towards Cys over the other amino acids studied.
In this report, we have successfully demonstrated that the aldehyde appended NDI is a selective sensor for Cys. The reaction between the aldehyde groups with Cys is responsible for the formation of a stable thiazolidine heterocycle. The Job plot clearly shows a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric ratio for receptor 1 towards Cys. The fluorescence emission of receptor 1 was quenched due to an photoinduced electron transfer from the nitrogen atom present in thiazolidine ring system. The optical properties and naked-eye color change of receptor 1 from yellow to colourless upon the addition of Cys in the presence of other amino acids showed a high selectivity towards Cys.
2 stoichiometric ratio for receptor 1 towards Cys. The fluorescence emission of receptor 1 was quenched due to an photoinduced electron transfer from the nitrogen atom present in thiazolidine ring system. The optical properties and naked-eye color change of receptor 1 from yellow to colourless upon the addition of Cys in the presence of other amino acids showed a high selectivity towards Cys.
Acknowledgements
S. V. B. (IICT) is grateful for financial support from the DAE-BRNS (Project Code: 37(2)/14/08/2014-BRNS), Mumbai, and Intelcoat project CSC0114, CSIR, New Delhi, India. SRB acknowledges the CSIR, New Delhi, for SRF support. R. S. B acknowledges financial support from the CSIR, New Delhi under the SRA scheme [13(8772)-A/2015-Pool]. S. V. B. (RMIT) acknowledges financial support from the Australian Research Council under a Future Fellowship Scheme (FT110100152) and the School of Applied Sciences (RMIT University) for the facilities. A. L. P. acknowledges the Gaussian 09 suite procured under the DST-FIST scheme (Sanction No. FS/FST/PSI-018/2009).Notes and references
- (a) Z. A. Wood, E. Schrӧder, J. R. Harris and L. B. Poole, Trends Biochem. Sci., 2003, 28, 32–40 CrossRef CAS PubMed; (b) J. B. Schulz, J. Limdenau, J. Seyfried and J. Dichgans, Eur. J. Biochem., 2000, 267, 4904–4911 CrossRef CAS PubMed.
- S. Shahrokhian, Anal. Chem., 2001, 73, 5972–5978 CrossRef CAS PubMed.
- V. Gazit, R. Ben-Abraham, R. Coleman, A. Weizman and Y. Katz, Amino Acids, 2004, 26, 163–168 CrossRef CAS PubMed.
- (a) G. L. Ellman, Arch. Biochem. Biophys., 1959, 82, 70–77 CrossRef CAS PubMed; (b) C. Komuro, K. Ono, Y. Shibamoto, T. Nishidai, M. Takahashi and M. Abe, J. Chromatogr., 1985, 338, 209–212 CrossRef CAS PubMed; (c) I. Daskalakis, M. D. Lucock, A. Anderson, J. Wild, C. J. Schorach and M. I. Levene, Biomed. Chromatogr., 1996, 10, 205–212 CrossRef CAS PubMed; (d) E. Kaniowska, G. Chwatko, R. Glowacki, P. Kubalczyk and E. Blad, J. Chromatogr. A, 1998, 798, 27–35 CrossRef CAS PubMed; (e) G. Chwatko and E. Blad, Talanta, 2000, 52, 509–515 CrossRef CAS PubMed; (f) T. Inoue and J. R. Kirchhoff, Anal. Chem., 2000, 72, 5755–5760 CrossRef CAS PubMed; (g) Y. V. Tcherkas and A. D. Denisenko, J. Chromatogr. A, 2001, 913, 309–313 CrossRef CAS PubMed; (h) P. Houze, S. Gamra, I. Madelaine, B. Bousquet and B. Gourmel, J. Clin. Lab. Anal., 2001, 15, 144–153 CrossRef CAS PubMed; (i) G. Chwatko and E. bald, J. Chromatogr. A, 2002, 949, 141–151 CrossRef CAS PubMed; (j) K. Amarnath, V. Amarnath, K. Amarnath, H. L. Valentine and W. M. Valentine, Talanta, 2003, 60, 1229–1238 CrossRef CAS PubMed; (k) N. Burford, M. D. Eelman, D. E. Mahony and M. Morash, Chem. Commun., 2003, 146–147 RSC; (l) D. Potesil, J. Petrlova, V. Adam, J. Vacek, B. Klejdus, J. Zehnalek, L. Trnkova, L. Havel and R. Kizek, J. Chromatogr. A, 2005, 1084, 134–144 CrossRef CAS PubMed; (m) Y. Sato, T. Iwata, S. Tokutomi and H. Kandori, J. Am. Chem. Soc., 2005, 127, 1088–1089 CrossRef CAS PubMed.
- (a) Y. Yue, Y. Guo, J. Xu and S. Shao, New J. Chem., 2001, 35, 61–64 RSC; (b) M. Zhang, M. Li, Q. Zhao, F. Li, D. Zhang, J. Zhang, T. Yi and C. Huang, Tetrahedron Lett., 2007, 48, 2329–2333 CrossRef CAS; (c) X. Zhang, X. Ren, Q.-H. Xu, K. P. Loh and Z.-K. Chen, Org. Lett., 2009, 11, 1257–1260 CrossRef CAS PubMed; (d) H. Wang, G. Zhou, H. Gai and X. Chen, Chem. Commun., 2012, 48, 8341–8343 RSC; (e) T.-K. Kim, D.-N. Lee and H.-J. Kim, Tetrahedron Lett., 2008, 49, 4879–4881 CrossRef CAS; (f) L. Duan, Y. Xu, X. Qian, F. Wang, J. Liu and T. Cheng, Tetrahedron Lett., 2008, 49, 6624–6627 CrossRef CAS; (g) Z. Yang, N. Zhao, Y. Sun, F. Miao, Y. Liu, X. Liu, Y. Zhang, W. Ai, G. Song, X. Shen, X. Yu, J. Sun and W.-Y. Wong, Chem. Commun., 2012, 48, 3442–3444 RSC; (h) F. Tanaka, N. Mase and C. F. Barbas III, Chem. Commun., 2004, 1762–1763 RSC; (i) M. Wei, P. Yin, Y. Shen, L. Zhang, J. Deng, S. Xue, H. Li, B. Guo, Y. Zhang and S. Yao, Chem. Commun., 2013, 49, 4640–4642 RSC; (j) K.-S. Lee, T.-K. Kim, J. H. Lee, H.-J. Kim and J. I. Hong, Chem. Commun., 2008, 6173–6175 RSC; (k) F. Kong, R. Liu, R. Chu, X. Wang, K. Xu and B. Tang, Chem. Commun., 2013, 49, 9176–9178 RSC; (l) A. Barve, M. Lowry, J. O. Escobedo, K. T. Huynh, L. Hakuna and R. M. Strongin, Chem. Commun., 2014, 50, 8219–8222 RSC.
- (a) D. Zhang, M. Zhang, Z. Liu, M. Yu, F. Li, T. Yi and C. Huang, Tetrahedron Lett., 2006, 47, 7093–7096 CrossRef CAS; (b) Y. Guo, S. Shao, J. Xu, Y. Shi and S. Jiang, Tetrahedron Lett., 2004, 45, 6477–6480 CrossRef CAS; (c) O. Rusin, N. N. S. Luce, R. A. Agbaria, J. O. Escobedo, S. Jiang, I. M. Warner, F. B. Dawan, K. Lian and R. M. Strongin, J. Am. Chem. Soc., 2004, 126, 438–439 CrossRef CAS PubMed; (d) W. H. Wang, O. Rusin, X. Y. Xu, K. K. Kim, J. O. Escobedo, S. O. Fakayode, K. A. Fletcher, M. Lowry, C. M. Schowalter, C. M. Lawrence, F. R. Fronczek, I. M. Warner and R. M. Strongin, J. Am. Chem. Soc., 2005, 127, 15949–15958 CrossRef CAS PubMed.
- (a) S. V. Bhosale, C. H. Jani and S. J. Langford, Chem. Soc. Rev., 2008, 37, 331–342 RSC; (b) F. Würthner and M. Stolte, Chem. Commun., 2011, 47, 5109–5115 RSC; (c) S. Bhosale, A. L. Sisson, P. Talukdar, A. Fürstenberg, N. Banerji, E. Vauthey, G. Bollot, J. Mareda, C. Rçger, F. Würthner, N. Sakai and S. Matile, Science, 2006, 313, 84–86 CrossRef CAS PubMed; (d) H. E. Katz, A. J. Lovinger, C. Kloc, T. Siegrist, W. Li, Y.-Y. Lin and A. Dodabalapur, Nature, 2000, 404, 478–481 CrossRef CAS PubMed; (e) S. V. Bhosale, S. V. Bhosale and S. K. Bhargava, Org. Biomol. Chem., 2011, 10, 6455–6468 RSC; (f) S.-L. Suraru and F. Würthner, Angew. Chem., Int. Ed., 2014, 53, 7428–7448 CrossRef CAS PubMed; (g) N. Sakai, J. Mareda, E. Vauthey and S. Matile, Chem. Commun., 2010, 46, 4225–4237 RSC.
- (a) T. Gunnlaugsson, P. E. Kruger, T. C. Lee, R. Parkesh, F. M. Pfeffer and G. M. Hussey, Tetrahedron Lett., 2003, 44, 6575–6578 CrossRef CAS; (b) S. V. Bhosale, S. V. Bhosale, M. B. Kalyankar and S. J. Langford, Org. Lett., 2009, 11, 5418–5421 CrossRef CAS PubMed; (c) M. R. Ajayakumar, S. Yadav, S. Ghosh and P. Mukhopadhyay, Org. Lett., 2010, 12, 2646–2649 CrossRef CAS PubMed; (d) D. Buckland, S. V. Bhosale and S. J. Langford, Tetrahedron Lett., 2011, 52, 1990–1992 CrossRef CAS; (e) S. Guha and S. Saha, J. Am. Chem. Soc., 2010, 132, 17674–17677 CrossRef CAS PubMed; (f) M. R. Ajayakumar, G. Hundal and P. Mukhopadhyay, Chem. Commun., 2013, 49, 7684–7686 RSC; (g) N. V. Ghule, R. S. Bhosale, K. Kharat, A. L. Puyad, S. V. Bhosale and S. V. Bhosale, ChemPlusChem, 2014, 80, 485–489 CrossRef.
- (a) S. R. Bobe, A. Gupta, A. Rananaware, A. Bilic, S. V. Bhosale and S. V. Bhosale, RSC Adv., 2015, 5, 4411–4415 RSC; (b) Y. H. Kim, S. G. Kwon and R. Kim, PCT Int. Appl., WO2014200249 A1 20141218, 2004.
- M. J. Frisch, et.al., Gaussian 09, revision. C.01, Gaussian Inc., 2009 Search PubMed.
- Avogadro: an open-source molecular builder and visualization tool. Version 1.1.0, http://avogadro.openmolecules.net/ Search PubMed.
- M. D. Hanwell, D. E. Curtis, D. C. Lonie, T. Vandermeersch, E. Zurek and G. R. Hutchison, J. Cheminf., 2012, 4, 1–3 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis and TEM measurements. See DOI: 10.1039/c5ra17809f |
| This journal is © The Royal Society of Chemistry 2015 |