Temperature-dependent oil absorption of poly(oxypropylene)amine-intercalated clays for environmental remediation†
Abstract
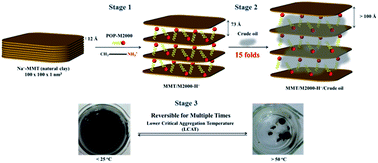
Natural silicate clays with layered structures were intercalated with polyether-amines via an ionic exchange reaction and subsequently applied for oil absorption. Polyether-amines, including hydrophobic poly(oxypropylene)-amines (POP-amines) and the hydrophilic poly(oxyethylene)-amine (POE-amine), were used to intercalate sodium montmorillonite (MMT). The organoclays obtained from POP-monoamine of 2000 g mol−1 (POP-M2000) demonstrated the ability to absorb petroleum crude oils in water at an efficacy of 15 times the weight of the organoclay. Owing to the property of lower critical aggregation temperature (LCAT) at 50 °C, the organoclays absorbed oils and self-aggregated into oil lumps and phase separated out from water cleanly above this temperature. By comparison, a homogeneous emulsion-like dispersion was formed at lower temperatures of 15 °C. This phenomenon was explained by the inherent property of lower critical solubility temperature (LCST) due to the presence of the POP-organics intercalated in the clay galleries. In addition to the POP hydrophobicity for highly efficient oil absorption, the LCAT behavior resulted in the ease of oil recovery under heating. The oil-absorbed clay aggregates were analyzed by XRD and showed the expansion and layer exfoliation of the clay structure during the incorporation of oil. The understanding of the POP-intercalated and oil-absorbed tertiary structures has led to their potential use in the areas of secondary oil recovery, shale oil extraction and remediation of oil pollution in water.

 Please wait while we load your content...
Please wait while we load your content...