An all solution-based process for the fabrication of superstrate-type configuration CuInS2 thin film solar cells
Abstract
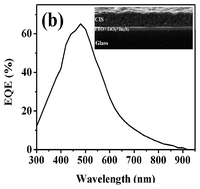
CuInS2 (CIS) thin films have proven to be promising candidates for photovoltaic technology but still the cost and safety of their fabrication processes remain challenging topics for research and development. Our efforts are based on avoiding the costly vacuum-based deposition methods that use selenization and high temperature processes. A cadmium free cell structure 〈glass/FTO/TiO2/In2S3/CIS/carbon〉 was fabricated using a CuInS2 nanocrystal ink at low temperature without selenization or sulfurization steps. All processes used have been non-vacuum and solution based. To form a stable ink, surfactant and binder-free monodispersed CIS nanocrystals were synthesized via a hot injection method in ethylene glycol solvent and re-dispersed in DMF. A spray pyrolysis method was used for the deposition of TiO2 and In2S3 as blocking and buffer layers, respectively. A doctor blade method was used to coat CIS films on the buffer layer. The final CIS absorber layer was achieved after heat treatments at 150 and 250 °C, without a selenization process. The amount of carbon residue in the final CIS film was very low (∼3%). Effective parameters on photovoltaic performance including the type of sulfur source, buffer-layer thickness and CIS grain size and morphology were optimized. The optimum superstrate-type solar cell device showed promising power efficiency of up to 3%.

 Please wait while we load your content...
Please wait while we load your content...