High brightness turquoise light-emitting diodes based on ZnO microwires†
Dengkui Wangabc,
Fei Wang*a,
Bin Zhaoac,
Yunpeng Wanga and
Dongxu Zhao*a
aState Key Laboratory of Luminescence and Applications, Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences, 3888 Dongnanhu Road, Changchun 130021, People's Republic of China. E-mail: wangf@ciomp.ac.cn; zhaodx@ciomp.ac.cn
bState Key Laboratory of High Power Semiconductor Lasers, School of Science, Changchun University of Science and Technology, 7089 Wei-Xing Road, Changchun 130022, People's Republic of China
cUniversity of The Chinese Academy of Sciences, Beijing 100049, People's Republic of China
First published on 14th October 2015
Abstract
ZnO microwire clusters have been fabricated by a chemical vapor deposition method on copper foil. Bright green luminescence was obtained when the sample was excited by an ultraviolet lamp. The mechanism of green luminescence and the relationship of point defect with emission were analyzed in detail. The quantum yield of green emission is 31%. Light emitting diodes were prepared based on ZnO microwires with a p-GaN or n-GaN film heterojunction. High brightness turquoise emission was obtained and the mechanism is discussed in this paper.
Introduction
Nowadays, high-brightness ultraviolet (UV), blue and green light emitting diodes (LEDs) are manufactured from the InAlGaN system, while high-efficiency yellow, orange, and red LEDs are made from the InAlGaP system.1,2 High efficiency green LEDs are in strong demand in display and solid state lighting, but the peak internal quantum efficiency (IQE) of green LEDs is significantly lower than that of shorter wavelength InAlGaN-based blue LEDs and longer wavelength InAlGaP-based red LEDs.3,4 For the GaN based green LEDs, the low IQE is due to the difficulty in growing high-quality high-In content InGaN alloys.5 Therefore, we explore new techniques or new materials to improve the performance of green LEDs.ZnO is a multifunctional material with different applications, such as phosphors,6 transparent conducting films,7 gas sensors,8etc. Recently, ZnO is considered as a potential material for the high efficient ultraviolet light emitting diodes9,10 and lasers diodes,11–13 due to the wide band gap of 3.37 eV and the large exciton binding energy of 60 meV at room temperature.14 ZnO based ultraviolet electroluminescence (EL) devices have been studied extensively,15,16 but the green EL devices have been barely studied.
In this paper, ZnO microwires with the high efficient green luminescence were fabricated using a chemical vapor deposition (CVD) method.17 To be constructed heterojunction with the n-GaN film, bright greenish blue EL could be obtained. The emission mechanism of these devices was analyzed.
Experimental section
ZnO microwires synthesis
ZnO microwires were synthesized from ZnO and carbon powders by the traditional CVD method. The mixture powders with weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were milled in an agate mortar to nano scale. And then, the mixture powders were transferred into a ceramic boat serving as the source material. A copper foil serving as substrate was placed right above of the powders with an appropriate distance (∼5 mm). The boat was placed into an alumina horizontal tube furnace and heated to 990 °C for 45 min using Ar (99.99%) as a carrier gas with the flow rate of 120 cm3 min−1.
1 were milled in an agate mortar to nano scale. And then, the mixture powders were transferred into a ceramic boat serving as the source material. A copper foil serving as substrate was placed right above of the powders with an appropriate distance (∼5 mm). The boat was placed into an alumina horizontal tube furnace and heated to 990 °C for 45 min using Ar (99.99%) as a carrier gas with the flow rate of 120 cm3 min−1.
Preparation of electroluminescence device
Previously reported ZnO/GaN heterojunction LEDs were constructed by growing ZnO on p-GaN substrates.18 In this work ZnO microwires were synthesized first, then sandwiched between ITO transparent electrode and GaN film. To provide Ohmic contacts, Ni/Au electrodes were prepared on the GaN surface by the metal thermal evaporation method under the pressure of 2 × 10−3 Pa. Before evaporation, GaN was ultrasonically cleaned with acetone, ethanol and deionized water for 10 min, respectively. Then, the as-grown ZnO microwires were transferred to the other part of ITO surface. To construct the LED, the GaN film was placed face to face on the ITO. Subsequently, they were fixed together by clips to form fine physical contact.Characterizations
The morphology of as-grown ZnO sample was investigated by a field emission scanning electron microscopy (FESEM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hitachi S-4800). The low temperature photoluminescence (LTPL) spectra measurements were carried out using a He–Cd laser of 325 nm as an excitation source with the micro-Raman spectrometer. The photoluminescence (PL) quantum yield in solid state was obtained in a calibrated integrating sphere using FL920 time-corrected single photon counting system (excitation wavelength: 325 nm). The PL of ZnO microwires and EL of the LED were performed by using fluorescence spectrophotometer (Hitachi F4500). The current–voltage (I–V) characteristic curve of the device was measured by the Keithley 2611A measurement system.
Hitachi S-4800). The low temperature photoluminescence (LTPL) spectra measurements were carried out using a He–Cd laser of 325 nm as an excitation source with the micro-Raman spectrometer. The photoluminescence (PL) quantum yield in solid state was obtained in a calibrated integrating sphere using FL920 time-corrected single photon counting system (excitation wavelength: 325 nm). The PL of ZnO microwires and EL of the LED were performed by using fluorescence spectrophotometer (Hitachi F4500). The current–voltage (I–V) characteristic curve of the device was measured by the Keithley 2611A measurement system.
Results and discussion
Flocculent-like ZnO clusters are obtained on a copper foil. Fig. 1(a) shows the typical field emission scanning electron microscopy image of ZnO microwires grown using the CVD method. The insert of Fig. 1(a) shows the FESEM image of a single microwire, which displays that the diameter of ZnO microwire is about 3 μm and the cross section is tetragonal rather than normal hexagonal. In order to exclude Cu doping in ZnO crystal lattice, the element composition is characterized by the energy-dispersive X-ray spectroscopy (EDX, as shown in another insert of Fig. 1(a)). The mole ratio of Zn element to O element is consistent with stoichiometric ratio of ZnO, and there are no copper signals to be detected. The X-ray diffraction (XRD) measurement is performed at room temperature to study crystal structure of the ZnO microwire, which is shown in Fig. 1(b). All the diffraction peaks observed are consistent with the ZnO wurtzite crystal structure, which suggests that the as-grown ZnO is wurtzite structure with the high crystal quality and no copper element is doped into the ZnO crystal lattices.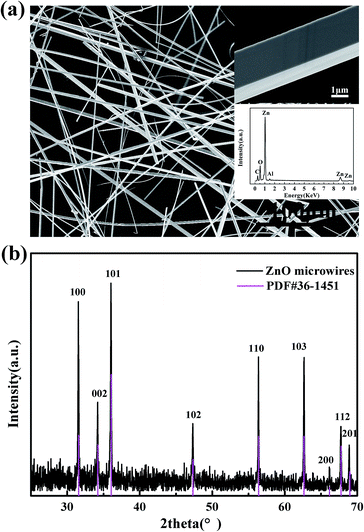 | ||
| Fig. 1 (a) The SEM image of ZnO microwire clusters; the insets are SEM image of single microwire and EDX spectrum, (b) XRD pattern of prepared ZnO microwire sample. | ||
The temperature-dependent PL spectra were measured ranging from −180 °C to 25.5 °C, as shown in Fig. 2. A strong wide emission peak from 450 nm to 610 nm is observed, while the near band edge emission of ZnO is so weak that almost cannot be observed. When microwires are excited by the 365 nm ultraviolet lamp with the power density of 1.21 mW cm−2, high-brightness green emission could be observed, as shown in the insert of Fig. 2.19 The other insert of Fig. 2 shows the low temperature near band gap PL spectra at −180 °C. The peaks at 366.7 nm, 374.5 nm are attributed to the free exciton (FE) emission and the neutral donor-related bound exciton transition,20 respectively. The emission peak originated from donor-related bound exciton provides evidence of the existence of oxygen vacancy. The quantum yield of green emission is 31%.
 | ||
| Fig. 2 LTPL of ZnO microwires excited by 325 nm He–Cd laser, the inserts are image of the light emission at room temperature and the band gap emission of ZnO microwires at −180 °C. | ||
In the preparation processes, six types of intrinsic defects may be present in ZnO nanostructure, respectively, VO, VZn, Oi, Zni, OZn, and ZnO. Among these point defects, Zni, ZnO and VO are considered as donors and the others are acceptors.14,21,22 The Zni, ZnO and VO generate shallow donors, while the Oi, OZn, and VZn form deep energy levels. These defects can result in blue, green and yellow emissions, but the mechanism of luminescence was not yet clear. For green luminescence, researchers have difference points about the mechanism.23–25 Most researchers consider the green emission originated from the transition of shallow donor energy electrons to valence band.26
In order to further reveal the mechanism of photoluminescence, the samples excited by the Xe lamp at various wavelengths using Hitachi F-4500 were measured, as shown in Fig. 3. The as-grown ZnO sample exhibits a remarkable emission with peak at 490 nm when the excitation wavelength is shorter than 380 nm. When the excitation wavelength exceeds 380 nm, the emission at 490 nm disappear, which suggests that the photon energy of excitation light must be larger than the energy gap of ZnO and the emission is associated with conduction electrons. It is concluded that the emission originates from the transition of oxygen vacancy donor energy electrons to valence band. The valence band electrons absorb the photon energy and leaps into conduction band. Subsequently, the excited state electrons transfer to VO donor level under the effect of relaxation, and then return back to the valence band, so the strong emission rather than the near-band emission is observed. When photon energy is lower than band gap, the ground state electron could not leap into the excited state. The conduction band has no electrons transit to oxygen vacancy energy level, so the emission is absent. Our experiment result is fully consistent with theoretical analysis.27 The excitation spectra to prove our presupposition in another way was measured (shown in insert of Fig. 3). The excitation peak is around 377 nm and exhibits a sharp drop, whose stopping is around 395 nm, which photon energy is approximately equal to the energy gap of ZnO at room temperature. The PL peak is hardly obtained when wavelength exceeds 395 nm. It is Similar to the results reported by other group.28
 | ||
| Fig. 3 The PL spectra of ZnO microwires excited by different excitation wavelengths. The insert is excitation spectrum of ZnO microwires. | ||
LEDs based on as-grown ZnO microwires have been constructed by using p-GaN. The Ni/Au electrode was prepared on p-GaN by the metal thermal evaporation method to obtain ohmic contact. The carrier concentration and mobility of p-GaN are 3 × 1017 cm−3 and 9 cm2 V−1 s−1, respectively. ZnO microwires contacted with p-GaN by using the physical method to avoid form ZnGaO interface layer.29 The schematic diagram of our device is shown in Fig. S1.† The typical I–V characteristic curve and the EL spectra of this device are shown in Fig. 4. The I–V characteristic of this device displayed typical rectification behavior. The forward threshold voltage was about 18 V. As can be seen in Fig. 4, only one emission peak centred at 395 nm was detected at room temperature with the voltage from 20 V to 28 V. The EL spectra intensity increases with the applied biases, but the peak position does not change.
 | ||
| Fig. 4 EL spectra under various forward biases of the device prepared by ZnO microwires and p-GaN thin film. The insets are I–V characteristics curve and image of ultraviolet emission of the device. | ||
To further study the performance of this device, Gaussian fitting was used to analyze the emission spectrum. Fig. S2† shows the Gaussian fitting results of emission spectrum under 28 V bias. The emission peak consisted of three parts. As previously reported, the peak centered at 410 nm comes from the interfacial radiative recombination of the electrons from n-ZnO and holes from p-GaN.30 The peak located at 392 nm is attributed to the NBE emission in ZnO, which originates from the recombination of ZnO free and bound exciton. The peak at 435 nm was considered from the transition of electrons on p-GaN conduction band to Mg doping level.31 The energy band structure of the p-GaN/ZnO device under forward bias is shown in Fig. S3.† Three arrows in the figure indicate the above mentioned three processes, respectively. Because the interface between ZnO microwires and p-GaN dominates the transition in the EL process, we obtained the ultraviolet emission instead of the green emission from the former device.
To obtained green emission, another heterojunction device was prepared by using ZnO microwires with the n-type GaN. The carrier concentration and mobility of n-GaN are 1.4 × 1019 cm−3 and 120 cm2 V−1 s−1, respectively. The schematic diagram of this device was similar with the former device, as shown in Fig. S1.† The typical I–V characteristic curve was measured using n-ZnO as anode, as shown in insert of Fig. 5. The forward threshold voltage was about 30 V. As can be seen in Fig. 5, the EL spectra of the device is detected at room temperature with the various biases, we can only find one emission peak at 490 nm. The wavelength is located at the border between green and blue. We depict it as “turquoise”. The intensity of turquoise emission is so great that could be observed by our naked eyes even in extraordinary bright environment. The other insert of Fig. 5 shows an image of our device with the voltage of 50 V. While the reverse bias was applied, there was no emission to be obtained.
 | ||
| Fig. 5 EL spectra under various forward biases of the device prepared by ZnO microwires and n-GaN thin film. The insets are I–V characteristics curve and image of turquoise emission of the device. | ||
The energy band diagram of this device is shown in Fig. 6. Under reverse bias, the ZnO conduction band and valence band increase and generate a barrier. Electrons in the conduction band of ZnO cannot pass through the barrier into n-GaN, which leads to the low forward current. There is no emission in this case.32 It is worth noting that the operating voltage of the device is much higher than that of the other device mentioned above. When the forward bias is applied, electron in the conduction band of n-GaN is accelerated and transfers to ZnO. The electron–hole pair is generated by the collisional ionization interaction of accelerated electrons. The generated electron transits to oxygen vacancy level and the hole stays in the valence band. Electron on oxygen vacancy level recombines with holes on valence band. Therefore, the turquoise emission can be obtained.
Conclusions
In summary, ZnO microwire clusters were fabricated on the copper foil by simple chemical vapor deposition method. Bright green luminescence originated from oxygen vacancy of the ZnO lattice was obtained when excited by 365 nm ultraviolet lamp. PL and excitation spectra proved that the emission was derived from the recombination of oxygen vacancy donor level electrons with holes on ZnO valance band. At last, turquoise emission was realized from LEDs prepared using n-GaN with ZnO microwires.Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program) under Grant No. 2011CB302004, the Key Program of National Natural Science Foundation of China under Grant No. 11134009, and the National Natural Science Foundation of China under Grant No. 21101146, 61307045, 61404009, 61474010, 61574022 and 61504012.References
- S. W. Feng, Y. C. Cheng, Y. Y. Chung, C. C. Yang, K. J. Ma, C. C. Yan, C. Hsu, J. Y. Lin and H. X. Jiang, Appl. Phys. Lett., 2003, 82, 1377–1379 CrossRef CAS PubMed.
- R. P. Schneider Jr, R. P. Bryan, J. A. Lott and G. R. Olbright, Appl. Phys. Lett., 1992, 60, 1830–1832 CrossRef PubMed.
- S. Nakamura, M. Senoh, S. Nagahama, N. Iwasa, T. Yamada, T. Matsushita, Y. Sugimoto and H. Kiyoku, Jpn. J. Appl. Phys., 1997, 36, L1059–L1061 Search PubMed.
- S. Nakamura, Science, 1998, 281, 956–961 CrossRef CAS.
- S. Nakamura, M. Senoh, N. Iwasa and S. Nagahama, Jpn. J. Appl. Phys., 1995, 34, L797–L799 CAS.
- K. Vanheusden, C. H. Seager, W. L. Warren, D. R. Tallant and J. A. Voigt, Appl. Phys. Lett., 1996, 68, 403–405 CrossRef CAS PubMed.
- Q. Wan, Q. H. Li, Y. J. Chen, T. H. Wang, X. L. He, J. P. Li and C. L. Lin, Appl. Phys. Lett., 2004, 84, 3654–3656 CrossRef CAS PubMed.
- J. H. Lee, K. H. Ko and B. O. Park, J. Cryst. Growth, 2003, 247, 119–125 CrossRef CAS.
- O. Lupan, T. Pauporté and B. Viana, Adv. Mater., 2010, 22, 3298–3302 CrossRef CAS PubMed.
- H. Lim, C. K. Kang, K. K. Kim, I. K. Park, D. K. Hwang and S. J. Park, Adv. Mater., 2006, 18, 2720–2724 CrossRef PubMed.
- H. Zhu, C. X. Shan, J. Y. Zhang, Z. Z. Zhang, B. H. Li, D. X. Zhao, B. Yao, D. Z. Shen, X. W. Fan, Z. K. Tang, X. H. Hou and K. L. Choy, Adv. Mater., 2010, 22, 1877–1881 CrossRef CAS PubMed.
- Z. K. Tang, G. K. L. Wong, P. Yu, M. Kawasaki and A. Ohtomo, et. al., Appl. Phys. Lett., 1998, 72, 3270–3272 CrossRef CAS PubMed.
- H. Kind, H. Q. Yan, B. Messer, M. Law and P. D. Yang, Adv. Mater., 2002, 14, 158–160 CrossRef CAS.
- Ü. Özgür, Y. I. Alivov, C. Liu, A. Teke and M. A. Reshchikov, et. al., J. Appl. Phys., 2005, 98, 041301 CrossRef PubMed.
- S. L. Zhao, P. Z. Kan, Z. Xu, C. Kong, D. W. Wang, Y. Yan and Y. S. Wang, Org. Electron., 2010, 11, 789–793 CrossRef CAS PubMed.
- C. Soci, A. Zhang, B. Xiang, S. A. Dayeh, D. P. Aplin, J. Park, X. Y. Bao, Y. H. Lo and D. Wang, Nano Lett., 2007, 7, 1003–1009 CrossRef CAS PubMed.
- P. C. Chang, Z. Y. Fan, D. W. Wang, W. Y. Tseng, W. A. Chiou, J. Hong and J. G. Lu, Chem. Mater., 2004, 16, 5133–5137 CrossRef CAS.
- S. Jha, J. C. Qian, O. Kutsay, J. J. Kovac, C. Y. Luan, A. J. Zapien, W. J. Zhang, S. T. Lee and I. Bello, Nanotechnology, 2011, 22, 24520 Search PubMed.
- F. A. Selim, M. H. Weber, D. Solodovnikov and K. G. Lynn, Phys. Rev. Lett., 2007, 99, 085502 CrossRef CAS.
- Z. Guo, D. X. Zhao, D. Z. Shen, F. Fang, J. Y. Zhang and B. H. Li, Cryst. Growth Des., 2007, 7, 2294–2296 CAS.
- D. C. Look, J. W. Hemsky and J. R. Sizelove, Phys. Rev. Lett., 1999, 82, 2552–2555 CrossRef CAS.
- D. C. Look, G. C. Falow, P. Reunchan, S. Limpijumnong, S. B. Zhang and K. Nordlund, Phys. Rev. Lett., 2005, 95, 225502 CrossRef CAS.
- S. A. M. Lima, F. A. Sigoli, M. Jafelicci Jr and M. R. Davolos, Int. J. Inorg. Mater., 2001, 3, 749–754 CrossRef CAS.
- H. B. Zeng, G. T. Duan, Y. Li, S. K. Yang, X. X. Xu and W. P. Cai, Adv. Funct. Mater., 2010, 20, 561–572 CrossRef CAS PubMed.
- B. X. Lin, Z. X. Fu and Y. B. Jia, Appl. Phys. Lett., 2001, 79, 943–945 CrossRef CAS PubMed.
- H. S. Kang, J. S. Kang, J. W. Kim and S. Y. Lee, J. Appl. Phys., 2004, 95, 1246–1250 CrossRef CAS PubMed.
- S. B. Zhang, S. H. Wei and A. Zunger, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 075205 CrossRef.
- X. Y. Xu, C. X. Xu, Z. L. Shi, C. Yang, B. Yu and J. G. Hu, J. Appl. Phys., 2012, 111, 083521 CrossRef PubMed.
- H. H. Huang, G. J. Fang, Y. Li, S. Z. Li, X. M. Mo, H. Long, H. N. Wang, D. L. Carroll and X. Z. Zhao, Appl. Phys. Lett., 2012, 100, 233502 CrossRef PubMed.
- D. K. Hwang, S. H. Kang, J. H. Lim, E. J. Yang, J. Y. Oh, J. H. Yang and S. J. Park, Appl. Phys. Lett., 2005, 86, 222101 CrossRef PubMed.
- R. J. Molnar, R. Singh and T. D. Moustakas, Appl. Phys. Lett., 1995, 66, 268–270 CrossRef CAS PubMed.
- P. L. Chen, X. Y. Ma, Y. Y. Zhang, D. S. Li and S. R. Yang, J. Electron. Mater., 2010, 39, 652–655 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Schematic diagram of device, Gaussian fitting of electroluminescence spectrum, energy band structures of the p-GaN/ZnO device. See DOI: 10.1039/c5ra17627a |
| This journal is © The Royal Society of Chemistry 2015 |

