Challenges and developments of self-assembled monolayers and polymer brushes as a green lubrication solution for tribological applications
Simon Watson
*a,
Mengyan Nie
a,
Ling Wang
a and
Keith Stokes
ab
aNational Centre for Advanced Tribology at Southampton (nCATS), University of Southampton, Southampton SO17 1BJ, UK. E-mail: s.watson@soton.ac.uk
bPlatform Systems Division, Dstl, Porton Down, Salisbury, Wiltshire SP4 0JQ, UK
First published on 14th October 2015
Abstract
Self-assembled monolayers (SAMs), after originally being investigated due to their functions in changing surface wettability, have been significantly developed over the years. Many types of SAMs have been developed on a variety of substrates. However their formation mechanism, rate and quality are found to be influenced by many factors. A range of SAMs including single- and multi-component are included in this review with focus on the nano and macro tribological properties. More recently, surface initiated polymer brushes, i.e. macromolecular assemblies attached to a substrate, have emerged to be an alternative and promising method for surface modification. The ability to tether these macromolecules to tribological contacts is key to their resistance to shear under loaded contacts. This review also covers atom transfer radical polymerisation (ATRP) and the role of this technique in developing new lubrication solutions. Particular care has been taken to include the development of lubrication solutions for silicon nitride due to the importance of this material as an engineering ceramic. This paper reviews the state-of-the-art development of SAMs and polymer brushes especially the potential opportunities and challenges in applying them in tribological contacts as a lubrication solution.
1 Introduction
Whenever two surfaces come into contact there will be energy wasted due to friction which will result in wear or degradation of the surfaces. In the privately owned passenger vehicle market as well as heavy goods vehicles and busses, Holmberg et al. state that a third of the energy from fuel is used to overcome friction.1,2 Significant areas of friction are from such components as the drive chain, engine and transmission, tires and brakes. One way to reduce fuel expenditure on friction is to utilise correct and efficient lubrication solutions. Current liquid lubrication solutions are generally in two classes; organomolybdenum compounds and organic friction modifiers.3,4 Organic friction modifiers include carboxylic acids/free fatty acids, alcohols, esters and amines.4 There are two accepted mechanisms that explain the mechanism of lubrication for organic friction modifiers. One mechanism is that the polar functional groups of the friction modifier adsorb onto the surface, much like self-assembled monolayers (SAMs), and the carbon chains form a barrier to prevent substrate–substrate adhesion thus lowering friction.3,5,6 The other model is semi-ordered viscous multilayers prevent contact.3,5,7 Free fatty acids are still used today to reduce friction, this is due to their ability to create closely packed monolayers.5,8 Long chain amines have also been reported in clutch systems, lubricated sliding contacts and MEMS devices.3,9,10 Molybdenum dithiocarbamates (MoDTC) have been introduced into engine oils since the 1950s as an antiwear additive, however, it was not until the 1970s where its application as a friction modifier was realised.3,11 MoDTC has been reported to form MoO3 resulting in high wear rates as MoO3 is abrasive.4,12 The decomposition of MoDTC produces MoS2 sheets that bond onto surface asperities therefore reducing friction.4,13–16 The role of zinc dialkyldithiophosphate (ZDDP) and MoDTC has been reported as the additives are shown to synergize well.3,4,16However, in the current economic climate with the ever increasing costs of purchasing new equipment there is a greater need for more effective lubricants. When combined with the fact that in more recent years there are new limits on the amount of sulphur and phosphorus that can be used in lubricating oils.17,18 The removal of sulphur and phosphorus is required due to the effect they have on catalytic converters and increasingly rigorous emissions regulations.19 The metal oxides of these elements are believed to come from the additives of lubrication and will block filters and reduce the effectiveness of catalysts in converters.19
SAMs are thought to be a new lubrication solution and the relatively simple procedure for SAMs has definitely inspired researchers to develop new lubrication solutions in order to meet new regulations. Lubrication systems for tribological contacts such as engines have had SAMs applied with good effect. The ability of SAMs to form on a variety of surfaces has attracted tremendous efforts to investigate applications of SAMs in surface engineering and tribological systems, such as artificial joints, orthopaedic implants, corrosion protection and friction reduction.20,21 Lubrication solutions based on SAMs have proved to be successful, especially for nanoelectromechanical systems and microelectromechanical systems (NEMS/MEMS), where normal lubrication methods are not suitable.22–24
Since SAM is typically only a few nanometres thick, its mechanical properties such as shear resistance may not be sufficient for frictional contacts.25,26 Polymer brushes could be considered to be a new lubrication solution. Polymer brushes can reduce friction to very low levels with increased resistance to shearing in addition to higher resistance to compressive forces. Controlled radical polymerisation techniques such as atom transfer radical polymerisations, have proven to produce high density, thick polymeric films capable of successfully reducing friction.27,28 Developments such as activators regenerated by electron transfer and surface attached initiators further strengthen the possibilities of polymer brushes. However these processes may be expansive to be easily adopted as an alternative to existing lubricants.
This paper reviews the development of SAMs and polymer brushes and their potential and challenges as a lubrication solution for tribological applications.
2 SAMs
Self-assembled monolayers (SAMs) are molecular assemblies formed spontaneously on surfaces by adsorption that are organised into ordered domains. SAMs were first reported by Zisman in 1946, where a monolayer was formed on a clean metal substrate to change the wettability of the surface.29 Unfortunately little recognition of SAMs was gained till 1983, when Nuzzo and Allara discovered that SAMs can be prepared on gold by adsorption of di-n-alkyl disulfides from a diluted solution avoiding the use of moisture-sensitive chemicals and crystalline metal surfaces.30 Over the past 30 years, a tremendous amount of research has been carried out to develop various SAMs and to investigate their functions and applications.This section describes the formation mechanisms of SAMs on solid surfaces, the factors that are found to influence the quality of SAMs, the techniques that have been used to characterise SAMs and the challenges and the state-of-the-art development of SAMs as a lubrication solution.
2.1 SAM formation mechanisms
SAMs can be formed on surfaces either from a solution or though vapour deposition, while the former is more popularly used. Having selected a particular chemical for a SAM, it is usually dissolved in an appropriate solvent before a clean substrate is immersed or dipped into the solution for the monolayer to ‘grow’. The growth rate and the structure of the SAM not only depend on the type of the molecule and the surface chemistry of the substrate, but also the concentration of the molecule and immersion time.17,31–34 Details of these factors will be discussed in the following sections.A number of typical SAM structures are shown in Fig. 1, illustrating some choices of the chemicals (e.g. a variety of head groups, chain/spacer types and functional/end groups) and the types of substrates being investigated.17,31–34
All SAMs are formed in a similar way, i.e. usually through various types of adsorption through head group–substrate interactions. The reactions between the molecule and the substrate can however be complicated, depending on the chemistry of the two. For example, the reactions of a trichlorosilane and trimethoxysilane with a hydroxyl group on a surface and their by-products are shown in eqn (1) and (2). It can be seen that the trichlorosilane SAM has a hydrochloric acid by-product while the trimethoxysilane produces a methanol. This has to be carefully considered when choosing SAMs for engineering applications especially when corrosion is a problem. In another example for thiols on gold, a two-step reaction takes place, shown in eqn (3) and (4).21 The thiol is firstly physisorbed onto the Au surface followed by S–H cleavage and chemisorption.
| R–SiCl3 + 3(–OH) → R–Si–O3– + 3HCl | (1) |
| R–Si(OCH3)3 + 3(–OH) → R–Si–O3– + 3CH3OH | (2) |
| R–SH + Au → R–SHPhysAu | (3) |
 | (4) |
Growth rate. As concluded by many researchers, the first minute of immersion has been found to be the most important time where SAMs grow at the highest rates. As Aswal et al. showed in their studies, an 85% coverage of an octadecyltrichlorosilane (OTS) monolayer on silicon was achieved within 50 s of immersion.36 In a similar study of OTS on silicon, Balgar et al. also achieved approximately 80% coverage within 1 minute of immersion.37 The reactivity of head groups has been found to affect their adsorption rate. For example, when OTS and octadecyltrimethoxysilane SAMs were formed on silicon nitride under the same conditions, a complete monolayer of OTS was formed after 5 minutes of immersion while the octadecyltrimethoxysilane took 120 minutes due to the more reactive head group of OTS.17 This is because the bond dissociation energies required for OTS to replace the groups attached to the silicon atom with OH groups prior to adsorption are much lower than that for octadecyltrimethoxysilane.38
The formation of SAMs for both thiols on gold and silanes on silicon is found to follow a same two-step process (illustrated in Fig. 2, using information from ref. 29 and 39).
(1) Step 1 is a fast linear growth step following the Langmuir adsorption model. The growth in this step is limited by head–substrate interaction but can achieve up to 85% of the maximum coverage and near the maximum achievable contact angle. The duration of this step can range from a few seconds to a few minutes depending on the concentration of the precursor molecules.
(2) Step 2 is a slow growing process and can take hours to complete. During this step, the SAM coverage plateaus and reaches to its maximum eventually. The maximum coverage is limited by adsorption from solution such as chain disorder interference.
During the self-organizing process, step 2, the molecules in SAMs rely on weaker and less directional bonds, such as ionic bonds, hydrogen bonds, and van der Waals interactions, to organize atoms, ions or molecules into ordered structure, with the molecules or ions adjusting their own positions to minimize the thermodynamic energy. That's to say, the kinetics and equilibrium of SAM formation involve a delicate interplay between molecule–solvent interactions, substrate–adsorbate interactions, non-bonded interactions between adsorbates, and intra-molecular interactions such as bond stretches, angle bends, and torsion. Both chemisorption and intra- and inter-chain non-bonded interactions (e.g., van de Waals, steric, repulsive, and electrostatic forces) contribute to the packing and ordering of molecules in SAMs. The conformation of the individual chains within the assembly, and their packing and ordering with respect to each other depend on the balance between inter-chain forces, the interactions with the surface, and the entropic effects as well.
Growth characteristics. Bierbaum et al. found that OTS monolayers appeared to grow from island like concentrations of SAM.35 Similarly, Balgar et al. and Aswal et al. found that OTS as well as other long chain SAMs grew in an island-like model but not short chain SAMs.36,37 This is known as island nucleation growth.
In another experiment, Bierbaum et al. found that propyltrichlorosilane reacted with a clean silicon wafer extremely quickly and therefore it was not possible to determine whether island growth had occurred, possibly due to the shorter chain length not obscuring other sites for SAM adsorption.35 They also found that propyltrichlorosilane did not achieve expected levels of contact angle for a CH3 terminated SAM and suggested that the disordered monolayer was formed by short chain SAMs, which have small van der Waals forces that were not sufficient to force chains into order.40
As shown in Fig. 3, the OTS SAMs were grown initially from nucleation points on the silicon wafer where single molecules attached to the substrate, subsequent growth from these molecules is visible in 20 s.37 The growth of a single island was indicative of diffusion limited aggregation (DLA). DLA is a process of aggregation formed by diffusion, where a mobile molecule will contact an already adsorbed molecule and form a cluster.41 As the cluster grows an irregular shape can be formed. Branched structure can also be formed due to the low probability of a molecule contacting the middle of the cluster. The irregular shape is known as a fractal shape and is related to DLA.37,42,43 However, adsorption in a solution can be considered to be a 3D adsorption model and the fractal shape may not be as pronounced. The cluster has been found to be the same height as the final monolayer, indicating that the molecules are “standing up” and held in ordered domains by van der Waals forces.37 After reaching to a certain island size, new islands will be formed rather than adding to the existing ones. This continues until the monolayer is complete. Fig. 3 shows the formation of an OTS monolayer from agglomerations to fractal shape to near full monolayer. Between 20 and 50 seconds it is clear that islands are growing, it is also notable that there are smaller islands appearing that are represented by small white dots. From 75 seconds the islands are seen to grow until they are indistinguishable from others. In excess of 90 seconds it would be expected that the monolayer would form in full.
 | ||
| Fig. 3 (A) OTS monolayer growth over time, from partial island growth to full monolayer. 10 μm × 10 μm sample on Si wafer. Reprinted with permission from ref. 37. (B) Is a line profile of the OTS monolayer clusters showing heights which coincide with the theoretical SAM height. Reprinted with permission from ref. 53. | ||
Apart from OTS,31,35–37,44–46 the island nucleation growth has also been seen in the SAM formation of C12 alkylsilane,47 3-aminopropyltrimethoxysilane,48 octadecylphosphonic acid,43,49 octadecyltrimethylammonium bromide,43 (3-glycidoxypropyl)trimethoxysilane,50 octadecylamine,51 and 6-(3-triethoxysilylpropylamino)-1,3,5-triazine-2,4-dithiol monosodium.52
Although thiols follow the same adsorption steps as silanes described in Fig. 2, they appeared to have different growth characteristics. Thiols are thought to go through a number of steps as illustrated in Fig. 4:21,54
 | ||
| Fig. 4 An illustration of thiol based SAM growth on gold from physisorption, covalent bonding, “standing up” to a complete ordered monolayer. | ||
(A) Thiol molecules are physically adsorbed onto the surface, see eqn (3).
(B) Thiol molecules are covalently attached to the substrate and are in the “lying down” phase, see eqn (4).
(C) As more molecules adsorb onto the surface, thiol molecules become denser and start the “standing up” phase.
(D) The complete ordered monolayer is formed as more molecules from solution adsorb.
2.2 Factors influencing SAM formation
The growth rate and structure of SAMs are found to be affected by a large number of factors such as substrate surface preparation and cleaning process, concentration of the chemical, type of solvent, immersion time, humidity, and substrate orientation etc.17,31–34 This section reviews influence of key factors on SAM formation.A standard surface cleaning procedure involves ultrasonic cleaning in different solvents to remove surface containments and blow drying to get rid of solvents residues and dusts, but an additional step of hydroxylation is usually needed for silicon-based or metal oxides substrates. During the ultrasonic cleaning, the commonly used solvents are toluene, isopropanol, chloroform, acetone, deionised water, and ethanol. A normal procedure would involve cleaning the surface in a polar then nonpolar solvent to remove as many residues as possible. After the surface is cleaned, it is rinsed in deionised water then dried in air, argon or nitrogen.17,55–62 For systems that require a highly hydroxylated surface to form high quality SAM a surface oxidation treatment is also employed using either piranha or plasma.
Piranha hydroxylation treatment uses a solution of concentrated sulphuric acid (98%) and hydrogen peroxide (30%) at a ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 4
3, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 3
1 or 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.31,32,44,46,52,55,57,58,62–76 Samples are typically immersed in the solution at a temperature between 60 and 90 °C for duration of 30 to 60 minutes, although majority of the studies chose to immerse their samples at 90 °C for 30 minutes. The piranha treatment has been found to be an effective method to create a good OH-terminated surface where very low water contact angles of less than 10° can be achieved.
1.31,32,44,46,52,55,57,58,62–76 Samples are typically immersed in the solution at a temperature between 60 and 90 °C for duration of 30 to 60 minutes, although majority of the studies chose to immerse their samples at 90 °C for 30 minutes. The piranha treatment has been found to be an effective method to create a good OH-terminated surface where very low water contact angles of less than 10° can be achieved.
The plasma process is conducted through an oxygen plasma treatment, where suitable hydroxylation can be achieved within a few minutes. Plasma treatment will leave silicon-based substrates hydroxyl terminated in significantly less time than piranha.66,77–79 Wu et al. concluded that 10 minutes plasma treatment on silicon wafers resulted in a water contact angle of less than 5° indicating a very good OH-termination coverage.80 Wiegand et al. also achieved similar contact angles on silica (100) surface using plasma treatment in less than 5 minutes.81
As an extra surface preparation, hydrofluoric acid (HF) is sometimes used as an etchant to remove native oxide layer for one of two reasons. For example, hydrofluoric acid can be used to etch silicon dioxide leaving the substrate hydrogen terminated. Some authors used this treatment to enable the attachment of monolayers directly to the silicon atoms.22,82,83 However, the monolayers formed are not strictly self-assembled as they require UV induced coupling or elevated temperatures.84–86 Another reason surface etching using HF is to reform an oxide layer with a consistent depth by controlling the immersion time in piranha. Wang et al. achieved roughness of less than 0.5 Å that allowed them to grow ultra-smooth monolayers.46
Although similar results are achievable from the piranha and plasma treatments, the former is more popular mainly due to the implementation of standard laboratory chemicals with a facile method. However, piranha is known to be a dangerous solution which requires careful handling and disposal. Any contact with organics can result in explosions and improper storage of waste in sealed containers have both resulted in injuries. Conversely, plasma treatment instruments add additional costs to processing substrates and for ultra-clean wafers it is known that clean room conditions are favourable. The influences of these techniques will be further discussed later in this review.
In addition to solvent choice, water content is of a particular concern, especially for silanes, since the presence of water is required for initial hydrolysis of trichlorosilane group. However, too much water present in the solvent leads to polymerization of OTS in bulk solution, which competes with the surface reaction of single alkylsilane molecules for the monolayer formation.88 McGovern et al. stated that the optimum water content in a solvent is 0.15 mg/100 mL.89 The authors also found that less moisture can facilitate the formation of well-defined monolayer on the silicon substrate but slower adsorption kinetics, sometimes resulting in an incomplete monolayer. In anhydrous conditions, even in an argon-filled glove box, water is still present as a layer adsorbed on the silicon oxide surface, which can assist hydrolysis of chlorosilanes but confine the reaction to the oxide layer.39,90
2.3 Characterisation techniques
Being an extremely thin layer of a few nanometers on a surface, it is difficult to characterise and quantify SAMs. Over the years, a range of techniques have been used to confirm the formation of SAMs and characterise SAM properties, summarised in Table 1. Among them contact angle measurements, atomic force microscopy (AFM), X-ray photoelectron spectroscopy (XPS) and scanning tunnelling microscopy (STM) are most commonly used.| Properties of SAM | XPS | AFM/STM | XRD | Ellipsometry | IR | Raman | SFG | UPS | LEED | SIMS | SEM | RBS | ISS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Note: SFG, sum frequency generation spectroscopy; UPS, ultraviolet photoelectron spectroscopy; LEED, low energy electron diffraction; SIMS, secondary ion mass spectroscopy; RBS, Rutherford backscattering spectroscopy; ISS, ion scattering spectroscopy. | ||||||||||||||
| Coverage | Overall coverage/thickness | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| Pinholes | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| Elemental composition | Average | ✗ | ✗ | |||||||||||
| Surface | ✗ | ✗ | ||||||||||||
| Conc. profile | ✗ | ✗ | ✗ | |||||||||||
| Functional groups | Bonding | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| Valence | ✗ | ✗ | ✗ | |||||||||||
| Orientation | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| Conformation | ✗ | ✗ | ✗ | ✗ | ||||||||||
| Average composition | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| Depth profile | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||
| Ordering | Pinholes | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| Long-range dislocation | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||
| Orientation | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||
| Interface ordering | ✗ | ✗ | ||||||||||||
| Substrate/SAM interface | Bonding | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||
| Pinhole | ✗ | ✗ | ✗ | |||||||||||
Contact angle measurements have been widely used in SAM characterisation due to its easy access, simplicity and low cost. Comparing the angles before and after surface modification provides an indication of whether and how well a modification has taken place. Some authors have made their own instruments due to the simplicity of the technique as well as carry out unique experimentation.98,99 Using a homemade instrument, Bormashenko et al. compressed the droplet with a precise moveable stage to study the Cassie–Wenzel transition. Contact angle calculations have also been utilised in surface energy calculations.93
For ultra-hydrophilic surfaces (<5°), water droplets may deform on the surface and it is difficult to obtain the contact angles.81,100,101 Therefore, other liquids may be selected, such as diiodomethane, 1-bromonaphthalene, formamide, glycerol, or ethylene glycol,93,102 to overcome this issue. However, different liquids will result in different contact angles; this is due to the difference in solid–liquid and liquid–liquid interfaces. Strong solid–liquid forces will result in the liquid spreading across a surface and therefore a low contact angle. Contrary, strong liquid–liquid interactions will cause liquids to stick together and therefore reduce contact angle. The surface tension at the solid–liquid interface is due to different intermolecular forces such as hydrogen bonding, polar interactions and acid/base interactions, for this reason changing probe liquid can alter the shape of the sessile drop. Janssen et al. studied 21 different probe liquids on 11 different SAMs in addition to oxidised silicon wafers, where the different interactions and subsequent contact angles were observed.103 For example, the contact angle for water and dichloromethane on a silicon wafer was found to be <10° and 14.4° respectively. It is worth noting that the authors also could not consistently fit contact angles below 10°.
For nano-tribological studies of SAMs contact mode AFM is routinely used. Contact AFM investigates the friction between the AFM tip and the SAM surface. It measures single asperity contacts with an ultra-sharp cantilever without being influenced by the effect of surface roughness.23,67,105 AFM is able to view both hard and soft surfaces in liquid as well as air, hence images of polymer brushes have been taken without collapsing.62,106 Fig. 6A shows a 3D AFM image where dodecane residues are clearly seen within a fully covered OTS SAM on a silicon substrate. Although created intentionally Checco et al. show that many solvents can leave residues on surfaces as shown in Fig. 6.107 Therefore, for high quality images to be taken these residues must be considered. Fig. 6B is an example of an OTS SAM formed on a silicon wafer with a uniform film approximately the known height of OTS.31 However, as this substrate was only immersed for 60 seconds it is unlikely that this is the smoothest SAM possible, see SAM formation kinetics. AFM has also been used to determine the thickness of SAM films by line profiles.31,108,109 Fig. 6C and D show an example of line profiling. Fig. 6C is multiply alkylated cyclopentane (MAC) deposited on top of a decyltrichlorosilane monolayer, the authors then concluded that the MAC layer was not uniform. MAC is thought to be a good lubricant due to a selection of desirable properties such as viscosity, volatility, pour point and thermal stability.110 The droplets measured by AFM sectioning can be seen in D, and the ellipsometry height measurements were found to coincided with AFM vertical difference measurements.108
 | ||
| Fig. 6 (A) Is an AFM image showing dodecane residues on an OTS monolayer. Reprinted with permission from ref. 107 Copyright 2006 American Chemical Society. (B) Is a fully formed OTS monolayer on a silicon wafer. Reprinted with permission from ref. 31. (C) Is a 2D image of multiply alkylated cyclopentane on top of a decyltrichlorosilane monolayer. Reprinted with permission from ref. 108. (D) Is the corresponding section analysis with the line profile and markers indicated on (C). | ||
 | ||
| Fig. 7 An STM image of two different phases of hexanethiol on gold. (a) Is a rectangular lattice structure and (b) is striped. Reprinted with permission from ref. 21. | ||
 | ||
| Fig. 8 XPS spectra of (a) a clean silicon wafer and (b) OTS SAM. Reprinted with permission from ref. 39. | ||
2.4 Achievements and applications of SAMs
SAM has shown growth in a number of applications especially in sensors such as pH sensors, organic and inorganic species detectors125,126 and biosensors.127–129 As mentioned above, SAMs have also been used as a lubrication solution in MEMS/NMEMs. Many SAM studies have been focused on their formation on silicon wafers, due to their well-defined surface morphology and extremely low surface roughness (Ra < 0.001 μm), which maximises the functions of SAMs and helps SAM characterisation. This is a particular interest of this review due to the authors' interests in silicon nitride surfaces.The type of SAM molecule limits the contact angle that is achievable. For example, with CH3-terminated SAMs on silicon, the reported water contact angles do not exceed 110–112° for hydrocarbon tails with more than 10 carbons. This is irrespective of the amount of chlorine atoms attached to the silicon head group.36,130 However, greater contact angles can be achieved by introducing fluorine groups and/or structuring surfaces. A facile method of increasing contact angles has been demonstrated by using (tridecafluoro-1,1,2,2-tetrahydrooctyl)-1-trichlorosilane and 1H,1H,2H,2H-perfluorooctyltriethoxysilane to form SAMs on silicon and nanofibrils with contact angles achieved at 120° and 130° respectively.131,132 This is because the CF3 groups in SAMs can reduce surface free energy, thereby increasing surface hydrophobicity. Song et al. demonstrated another approach to increase contact angles to the hydrophobic range by coupling SAMs with micro-roughened surfaces.133
Apart from single molecule type SAM, mixed SAMs have been developed to incorporate two types of molecules in one SAM where one type of species would preclude the other by steric hindrance.91 Mixed monolayers have great potential in many different applications including microelectrodes,134 separation of biomolecules,135 environmental monitoring,136 biosensors137 and tribology.63 Feng et al. developed a mixed SAM containing both OTS and octyltriethoxysilane, see a schematic of the mixed SAM in Fig. 9D.65 Two methods were used to produce the mixed SAM: co-adsorption and stepwise as illustrated in Fig. 9A–C. Co-adsorption is considered the easier of the two methods, where a clean substrate was simply immersed in a solution containing two precursor molecules, Fig. 9A. Due to the different affinities of the molecule headgroups, typically the ‘trial and error’ approach is required to tailor the concentration and ratio between the two types of molecules to achieve the desired mixed SAM.65 It was found not possible to form a mixed SAM using OTS and octyltriethoxysilane but possible for OTS and dodecyltrichlorosilane.138 This was due to the steric hindrance of the three ethoxy groups in addition to the quick binding of OTS to the substrate rendering octyltriethoxysilane unable to form structures.65
 | ||
| Fig. 9 Showing an idealistic structure for the mixed SAM of OTS and octyltriethoxysilane. Reprinted with permission from ref. 65. | ||
The stepwise method is illustrated in Fig. 9B and C, this method was developed to overcome the problems of mixed co-adsorption. Firstly, a partial monolayer was produced by immersing a substrate in a solution of the first SAM forming molecules for a known length of time. Then the substrate was immersed in a secondary solution of a different molecule.48 Gaps in the first monolayer were filled in by the second SAM-forming molecule.48,65 It was recommended that the SAM with a larger head group should be formed first as the smaller steric hindrance of the secondary SAM will allow it to fit in the gaps left by the larger SAM.65 Gradient mixed SAM can be created by pumping toluene at a constant rate into a solution of OTS with a partially immersed substrate.63 With the addition of more solvent the solution is more dilute meaning longer adsorption times for a full monolayer.31,63 This is facilitated by a partially immersed substrate where the addition of more solvent increases solvent level and therefore immerses more substrate. After removal of the partially formed monolayer from one solution, simple immersion in another solution containing (1-trichlorosilyl undecyl)trichloroacetate in toluene creates the full monolayer.63 However, it has been found that in some cases phase separated islands of monolayer have been formed rather than well mixed monolayers. As previously discussed, the formation of mixed SAMs can be inhibited by molecules selected and therefore multistep adsorption may be required. This could limit applications.
Bilayers and multi-layered SAMs are increasingly seen in research with self-assembling multilayers incorporating other layers such as gold nanoparticles.139 Extensive research of bilayers has included self-assembling supported lipid layers, e.g. phospholipid bilayers are found to be useful in studying interactions with cell membranes.140,141 Supported bilayers of lipids are commonly produced by spreading lipid vesicles on hydrophilic solid supports.142 To create tethered bilayers authors have formed SAM of thiolipids on gold followed by the lipid bilayer below the critical micellar concentration, i.e. below the point at which micelles forms in solution.143 Bilayers of thiols and silanes can be formed depending on end group, thiols such as 11-mercapto-1-undecanol will allow silane SAMs to form a bilayer on top although.144 It is worth noting that –OH functional groups on the terminal end of SAMs are not as efficient as substrate groups at creating additional monolayers.144 OTS has shown the ability to form multilayers in poor solvent on both steel and silicon, however, it is reported that the layers were found to be extremely rough indicating a consistent multilayer has not been formed.53,145 In addition, authors that have used MAC,70,108,110 apply the mobile lubricant through spin coating possibly limiting applications in some areas.
2.5 SAMs for tribological applications
 | ||
| Fig. 10 (A) An illustration of four friction traces detailing the COF of different SAMs obtained from pin on disk experimentation. (B) Is a simplified standard model of friction (A) is reprinted with permission from ref. 56. | ||
2.6 SAMs on silicon for COF reduction
OTS SAM. OTS SAMs have been extensively investigated for tribological performance improvement of silicon wafers in a wide range of load and speed.63,71,75,162–164 Using a steel ball and forming OTS SAMs on silicon wafer disks, Cha et al. recorded COF values in the range of 0.1 to 0.2 over a dry sliding distance of 75 m under a load of 50 mN for immersions in excess of one hour. No damage was found on the wafer discs for longer immersion compared with the wear tracks under 10 s and 1 h immersion. The authors found that immersion times of longer than 5 hours had little effect on the COF recorded, however relative humidity proved to influence COF. A higher humidity resulted in a higher COF. Booth et al. found that the tribological properties of single-component OTS monolayer were dependent upon the surface coverage and surface energy of the gradient monolayer.63 They also demonstrated that the COF can be further reduced to under 0.1 on the OTS SAM fully covered silicon wafer under 98 mN loading, similar to the studies by DePalma et al.163 Satyanarayana et al. also demonstrated that with a stainless steel ball, COF of OTS SAM modified silicon disc can be reduced to 0.06 under 0.5 N compared to unmodified silicon (0.2–0.3).75 Ma et al. also performed similar tests under a load of 0.5 N, but found the COF stabilised at 0.13 on the OTS SAM modified silicon.70 However, the authors found the OTS SAMs instantly breakdown and failed under a higher load of 1 N. Garcia-Parajo et al. studied OTS SAMs on silicon with force distance curves and compressive forces, and proposed that rearrangement of OTS under compression and the subsequent reformation after load was removed contributes to the lubricity of OTS SAMs.165 Flater et al. demonstrated that OTS SAMs can reduce adhesion of silicon wafers through pull off measurements using AFM, and also noticed that friction is reduced further when OTS SAMs are created on both the cantilever and substrate.164
Influence of chain length and head group. A number of studies have been conducted to investigate the influence of chain length and head group type on their tribological properties. Singh et al. studied trichlorosilanes with 6, 10 and 18 carbons chain lengths using a reciprocating ball on plate configuration with a silicon nitride ball under a load of 4 mN.166 At a 1 mm s−1 sliding speed over a 3 mm track OTS SAMs produced a COF of less than 0.1, whereas DTS and HTS produced COF values of approximately 0.15 and 0.25 respectively. The performance of the three SAMs was also compared using a contact AFM under 40 nN and 2 μm s−1 scan rate. Their performance in terms of friction was ranked as OTS > decyltrichlorosilane (DTS) > hexyltrichlorosilane (HTS), where OTS had the lowest COF. Masuko et al. performed similar investigations using 6, 10, 14 and 18 carbon chain lengths and found a COF of under 0.1 for OTS SAMs using ball on disk under 52.1 mN load at a sliding speed of 3.53 mm s−1. Longer chain SAMs have been shown to have significant freedom of swing allowing rearrangement to the direction of shear stress, thus lowering COF.56,70,166
By replacing one or two Cl atoms with CH3 group, Masuko et al. also investigated effect of head groups on tribological performance of trichlorosilane SAMs on silicon.56 When a Cl atom was replaced an increase of COF was observed. Further increase of COF was shown when two Cl atoms were replaced. The authors concluded that replacing Cl atom of trichlorosilane with CH3 group increased steric hindrance, thereby making the formation of a dense monolayer difficult as well as the loss of cross linking between silicon atoms, which have an influential effect on stability and wear life of SAMs.
Other SAMs. DePalma also studied undecyltrichlorosilane (UTS) and (tridecafluoro-1,1,2,2-tetrahydrooct-1-yl)trichlorosilane (FHOTS) SAMs on silicon wafers, however, found that friction was higher than OTS SAMs in both cases, possibly due to the thicker films of 25 Å produced by OTS SAMs as opposed to 15 Å and 10 Å of UTS and FHOTS SAMs, respectively.163 Satyanarayana et al. also studied 3-aminopropyltrimethoxysilane (APTMS), however, APTMS SAMs did not show any lubricating properties.75 The increase in COF compared to a bare wafer is likely to be due to the hydrophilic terminal group producing a higher adhesive force which increases friction. However, Li et al. investigated tribological properties of 3-aminopropyltriethoxysilane (APTES) and phosphorylated APTES SAMs on silicon wafer using contact mode AFM under 20 nN load at 10 Hz scan rate, and observed that the COF was reduced from approximately 0.08 on bare Si wafer to around 0.03 on both APTES SAM and phosphorylated APTES SAM.68 Kang et al. prepared a 6-(3-triethoxysilylpropylamino)-1,3,5-triazine-2,4-dithiol monosodium (TES) SAM on silicon, and investigated its tribological performance using ball on disk with a 4 mm steel ball under a 0.098 N load. The authors reported a lower COF value of 0.12 compared to bare silicon which kept stable for 130 s or approximately 300 cycles. The reduction of friction on TES SAMs was claimed to be the van der Waals forces between the terminal groups on the ring structures.52
Booth et al. used OTS and (1-trichlorosilyl undecyl)trichloroacetate to create a dual-component gradient monolayer film on a silicon substrate.63 Dry sliding tests with a ball-on-plate microtribometer demonstrated that these gradient SAM films exhibit excellent lubricating performance with significant reduction in friction in comparison to bare Si substrate. By creating dual-component monolayers with methyl and hydroxyl terminals, greater durability was achieved on the mixed SAM films with constant lubricating performance over 5 h at a sliding speed of 0.1 mm s−1. The enhancement in tribological performance durability was proposed to stronger intermolecular interactions within dual-component mixed SAMs. Singh et al. observed contrary effect on mixed SAMs of OTS/DTS and OTS/HTS using microtribological tester with the deteriorated performance for both mixed SAMs compared to single component OTS SAM.166 However, under nanotribological testing conditions using AFM, both mixed SAMs outperformed the single component SAMs, which was postulated that the difference in carbon chain length provided less resistance as the AFM tip slides over the outer layer.
Satyanarayana et al. also investigated tribological properties of a composite film of OTS onto APTMS SAM. The authors demonstrated that the composite film exhibits lower COF and longer wear life than APTMS SAM, but nearly the same performance as OTS SAM. This film lasted slightly fewer cycles at equivalent COF to that of the OTS SAM before breaking down at 3000 cycles.75 Ma et al. created dual-layer films OTS/multiply-alkylated cyclopentane (MAC) and tested them using a ball on plate tribometer with a steel ball at a sliding speed of 1.5 mm s−1 under 0.5 and 1 N loads. OTS/MAC dual-layer films maintained a similar COF to OTS SAM through the 0.5 N testing but improved wear resistance through higher loaded tests. The improvement was attributed to that the MAC layer is able to reorganise and replenish the lubricant deprived area.70,108 Ren et al. prepared polyethyleneimine (PEI) SAM as well as a PEI–STA dual-layer film on silicon substrate by immersing the PEI modified substrate in a solution of stearic acid (STA) and N,N′-dicyclohexylcarbodiimide (DCCD).72 Using AFM and a unidirectional ball-on-plate tribometer, the authors demonstrated that the PEI SAM alone was not a good coating and only lasted a few cycles under low load. However, the PEI–STA dual-layer film shows much better tribological performance with a COF of 0.06 under the same load lasting for approximately 8600 cycles. The PEI SAM was not effective to reduce friction due to its minimal chain length and hydrophilic terminal group, while better performance of the PEI–STA film is possibly due to the long chain length of stearic acid which is able to rearrange the carbon chain in the shear direction and provides protection to wear. Yang et al. used (3-mercaptopropyl)trimethoxysilane (MPTS) to form a SAM on silicon followed by immersion in a silver nanoparticle solution resulting in a MPTS SAM doped with Ag. The doped layer was immersed in an octanethiol solution to form a sandwich-like trilayer film. Nanotribological testing performed using AFM under a load of 20 nN demonstrated that compared with the bare Si wafer, MPTS SAM reduces the frictional force by 3.5 times, Ag-doped MPTS SAM further reduce by more than 40%, and tri-layer film further reduces additional 15%. The authors proposed that the long tails of the octanethiol are able to pivot and therefore rearrange with the sliding direction of the AFM tip reducing the frictional force. Using a reciprocal tribometer with a load of 0.5 N at a sliding rate of 2.5 mm s−1, it is observed that under the selected macro-sliding conditions, the MPTS SAM is not suitable for surface protection of Si substrate with high COF as bare Si and a short film life of 200 s. However, improvement was evident after the SAM was doped with Ag nanoparticles and additional layer. The silver doped MPTS SAM exhibited an extended antiwear life of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s with a COF of 0.19, while the trilayer film showed over doubled lifetime of 29
000 s with a COF of 0.19, while the trilayer film showed over doubled lifetime of 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 s with a further reduction in COF of 0.16.74
500 s with a further reduction in COF of 0.16.74
To summarise, the most successful SAMs have shown to be longer chain SAMs and multilayers. However, multilayers inherently have their own problems with application to tribological contacts apart from NEMS and MEMS. This area appears to be where research is focussed with SAMs proving their worth, notably OTS. The majority of SAMs reviewed in this article, single, mixed or multiple, have been application driven with regards to NEMS/MEMS with exceptions for DePalma's earlier work which was purely interested in the tribological properties.
2.7 SAMs on silicon nitride
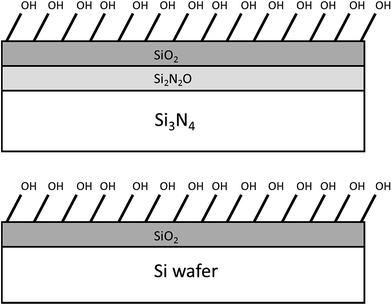
Sung et al. showed that monolayers of octadecyldimethylchlorosilane (ODS) could be formed on silicon nitride.182 After treating with HF, the monolayers were formed on silicon nitride from a solution of ODS in mixed solvents of hexadecane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform (4
chloroform (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for one hour. Water contact angle of 110° are obtained on the ODS SAM modified silicon surfaces, which are similar to those reported for silicon oxide, showing a good monolayer was formed on silicon nitride surface.182,183 This study also demonstrated that the monolayers can attach directly to H-terminated silicon nitride as well as the naturally found silicon oxide. With piranha treated silicon nitride good quality SAMs can also be formed on the oxide layer, as Kölbel et al. demonstrated the SAMs of chlorosilanes and ethoxysilanes formed on the piranha-treated silicon nitride exhibited equivalent contact angles to those SAMs formed on silicon wafers.184 Stability testing by washing with solvent, storing in water, heating or storing in ambient conditions for months did not affect the contact angle.184 Diao et al. successfully produced monolayers of APTES on silicon nitride with contact angles of ±5° comparable to that obtained on silicon wafers by Janssen et al.103,185 Wang et al. investigated SAM formation on silicon nitride using four silanes; OTS, octyltrichlorosilane, chlorodimethyloctadecylsilane and octadecyltrimethoxysilane.17 It was found that OTS and octadecyltrimethoxysilane achieved the highest contact angle of 108°. Like Kulkarni et al., the authors found that the initial formation was very quick and 80–90% of the monolayer is formed within an order of minutes.31
1) for one hour. Water contact angle of 110° are obtained on the ODS SAM modified silicon surfaces, which are similar to those reported for silicon oxide, showing a good monolayer was formed on silicon nitride surface.182,183 This study also demonstrated that the monolayers can attach directly to H-terminated silicon nitride as well as the naturally found silicon oxide. With piranha treated silicon nitride good quality SAMs can also be formed on the oxide layer, as Kölbel et al. demonstrated the SAMs of chlorosilanes and ethoxysilanes formed on the piranha-treated silicon nitride exhibited equivalent contact angles to those SAMs formed on silicon wafers.184 Stability testing by washing with solvent, storing in water, heating or storing in ambient conditions for months did not affect the contact angle.184 Diao et al. successfully produced monolayers of APTES on silicon nitride with contact angles of ±5° comparable to that obtained on silicon wafers by Janssen et al.103,185 Wang et al. investigated SAM formation on silicon nitride using four silanes; OTS, octyltrichlorosilane, chlorodimethyloctadecylsilane and octadecyltrimethoxysilane.17 It was found that OTS and octadecyltrimethoxysilane achieved the highest contact angle of 108°. Like Kulkarni et al., the authors found that the initial formation was very quick and 80–90% of the monolayer is formed within an order of minutes.31
Nanotribology. The majority of AFM tips are made of silicon nitride with pyramidal contact. SAMs have been applied to AFM tips to create chemical force microscopy (CFM),186 which is used to measure frictional response between the cantilever modification and substrate. The modified tip is useful for mapping different chemical functionalities across a substrate through the surface–tip interactions as some SAMs will result in different frictional responses. In some cases the cantilevers are first coated in gold then thiols are adsorbed, silanes have also been reported.187 The gold layer can be detrimental to friction experiments as the gold is weakly attached to the silicon nitride so for nanotribology direct attachment to silicon nitride is preferable.187 Adhesion testing is common with CFM, it can simply be explained as the force needed to retract the cantilever from the surface. Adhesion force measurements are related to the modified tip and surface interactions and can be used to measure stiction and combat adhesion related failures notable in NEMS/MEMS.188–190 Direct comparison of adhesive forces can be misleading if different tip radii are present.187 This is even more important if colloidal AFM cantilevers are used.191 Ito et al. found that adhesion force of CFM probes is in agreement with the increase of contact angle of the same SAM on a planar silicon nitride substrate.192 Headrick et al. shows an increase in pull off force when OTS SAM are applied to silicon nitride cantilever on a CH3 terminated substrate.193 OTS SAM cantilevers on COOH terminated surfaces show a slight increase in adhesion force in comparison to cleaved mica, this gives the unique ability to identify localised chemical groups on the surface through CFM through differences in pull off forces.193 Tsukruk et al. modified both cantilever and substrate and found that larger force displacement curves are recorded with CH3–CH3 terminations rather than Si3N4–Si3N4 contacts.187 Frisbie et al. modified gold coated silicon nitride cantilevers with both COOH and CH3 terminating SAMs.194 Fig. 12A shows a representation of the lithographically defined SAMs. Fig. 12B and C show the frictional response of a CH3 terminated cantilever and a COOH cantilever, respectively. Brighter areas are areas of higher friction.
 | ||
| Fig. 12 Representations of frictional force between a modified substrate shown in (A). (B) Is where a CH3 terminated cantilever is used and (C) is with a COOH terminated tip. Brightness is related to areas of higher friction. Adapted from Frisbie et al. and Barattin et al. Reprinted with permission from ref. 194. | ||
Macrotribology. An extensive literature search resulted in very little with regards to silicon nitride modified with SAMs. The authors found one publication reporting the results on SAMs on silicon nitride at macro scale. Wang et al. investigated SAM the tribological properties of two SAMs, OTS and octadecyltrimethoxysilane on silicon nitride.17 OTS is discussed here as it was a superior friction reducer. However, as the authors also pointed out, although octadecyltrimethoxysilane is not as good in reducing the COF as OTS, it may be more appropriate to use it instead of OTS as an additive for hybrid contacts, as there are fewer corrosive by-products than OTS (see eqn (1) and (2)). Tribological testing was conducted using a ball on disk tribometer, where the COF of a SAM modified disk was compared to that of an unmodified disk under a 10 N load and at a constant speed of 0.198 m s−1. Under dry conditions the OTS SAM produced a COF of 0.020 for 11.6 m before failure. When lubricated with a base oil the COF is reduced to 0.014. Interestingly the authors discovered that the best COF result comes from using OTS as an additive in a base oil, at concentrations of 2.5, 5 and 10 mM were tested. After 1250 m sliding the COF has settled to a steady value of 0.008 for all three solutions.
2.8 SAMs on metallic substrates and tribo-applications
Apart from on silicon wafer and silicon nitride, SAMs have also been developed on many other substrates such as alumina, iron oxide, steel, zinc, copper, platinum, silver, Ti6Al4V alloy, as well as gold, palladium, silver, copper and zinc. However their applications in tribology are still limited.91 This section reviews the development SAMs on metallic surfaces based on the head groups of the SAMs.OTS has also been attached to amorphous alumina and the kinetics as well as resulting monolayer are shown to be very similar to that of a silicon oxide substrate. Facilitated by hydroxylated surfaces SAM growth occurs with reported contact angles of 100°. However, the authors did not achieve known contact angles with Qin et al. and Wang et al. achieving considerably higher ≈120°.195,196
Qin et al. investigated the performance of octadecyltriethoxysilane, 1H,1H,2H,2H-perfluorodecyltriethoxysilane (PFDS) and APTES monolayers on aluminium alloys117 using a reciprocating ball on plate test rig. A steel ball was used as the counter surface. APTES failed instantaneously possibly due to short chain length not preventing contact but also due to the head group. PFDS successfully reduced the COF under loads of 0.6, 1 and 2 N for sliding times of 600 seconds, but failure occurred at approximately 400 seconds under 3 N load. Octadecyltriethoxysilane reduced the COF the most and completed all of the tests as well as a 4 N test without failure, the authors concluded the same mechanism for lubricity exists on aluminium as it does on silicon. The long chain coupled with the ability to swing as well as inter-chain interactions all help to reduce COF.117 Similar results were achieved by Devaprakasam et al. when 1H,1H,2H,2H-perfluorooctadecyltrichlorosilane (FOTS) and OTS were formed on aluminium and tested on both nano-and macrotribometers.94
Panjwani et al. formed 3-glycidoxypropyltrimethoxysilane on Ti6Al4V alloy, and then coated a perfluoropolyether (PFPE) layer on top of the preformed SAM. They tested the treated alloy against a silicon nitride ball on a ball on disk tribometer under 0.2 N, 41.9 mm s−1 and dry sliding conditions. It was found that the PFPE overcoat lasted over 900 times longer than without the coat due to its self-repairing characteristics.197 Li et al. created an APTES SAM on titanium alloy followed by a self-assembling graphene oxide (GO) layer as illustrated in Fig. 13.198,199 Reduced graphene oxide (RGO) sheets are prepared by heating GO sheets after assembly.
 | ||
| Fig. 13 Showing the interaction between the pin and APTES film and the ideal interaction with the GO/RGO sheets. Reprinted with permission from ref. 199. | ||
The modified Ti surfaces were then tested using a reciprocating pin on plate tribometer under dry conditions against a silicon nitride ball under 100 mN load and 1 Hz frequency. The APTES SAM showed a poor wear life and was destroyed within 500 seconds. The graphene oxide/APTES film however showed a COF of 0.19 and a life time of over 5000 seconds. This was further improved after heat treatment, where a COF of 0.16 and a life of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 seconds were achieved. It was suggested that it was due to less oxygen groups in the heat treated graphene layer.199
000 seconds were achieved. It was suggested that it was due to less oxygen groups in the heat treated graphene layer.199
 | ||
| Fig. 14 Illustrations of the formation of carboxylic acid monolayers (A and B) and the bonding characteristics of alkylphosphonic acid on copper oxide. Information from ref. 200 and 202. | ||
Wan et al. created SAM of alkylphosphonic acids on copper surfaces, where dodecylphosphonic (C12PA) and octadecylphosphonic (C18PA) acids were chosen. Ball on plate reciprocating tests with 5 mm steel balls under a load of 0.5 N and sliding velocity of 10 mm s−1 were performed. The C12PA SAM remains stable for approximately 50 seconds before failure and the C18PA SAM lasts more than 500 seconds before slow failure. This follows the same rules as silanes with regards to length of chain providing increased lubricity and wear life. Etching the copper surface using NaOH before SAM formation greatly improved the wear life of the films, wear life of the C18PA SAM formed on the etched copper substrate exceeded 7200 seconds under a COF of 0.2. Etching also increases the contact angle of both C12PA and C18PA SAMs significantly which may play a part in friction reducing.202 The tribological application of both C12Pa and C18PA on alumina was also investigated by using a reciprocating ball-on-flat microtribometer under dry conditions. In addition it is shown that both acids can reduce the COF, however, the longer chain length reduced friction more which is consistent with the literature.148
Zhang et al. investigated stearic acid (SA) SAM films on textured aluminium using a ball-on-disk tribometer with steel ball counterparts dry sliding at 10 mm s−1 under 0.1 N load. Surface texturing was completed using NaOH at 100° for 1 h, etching alone reduced the COF to below 0.6 from over 0.7 for non-etched Al surface. SA SAM on non-etched Al substrates reduced the COF to below 0.5, while the SAM on the etched substrates the COF was further reduced to under 0.15. The authors cited pivoting and rearrangement of the monolayer as an explanation for the reduction of COF.203 As Wan et al. observed,202 Zhang et al. also concluded that SAM modification combined with chemical etching can significantly reduce friction with drastically increased durability, and low friction of the combined modification is due to the reduction in surface adhesion between steel ball and the films as well as the actual contact areas.203 Carboxylic acids have been used to lubricate steel and diamond like carbon (DLC), in research completed by Simič et al. palmitic acid was the acid of choice. In the tribological tests palmitic acid was premixed into a poly alpha olefin base oil, testing was performed using a ball on flat reciprocating tribometer with steel on steel contacts or DLC on DLC at 25 °C and 80 °C with a load of 10 N at an average velocity of 0.01 m s−1. Steel on steel testing at the lower temperature shows that the COF is reduced from approximately 0.15 to 0.10 for the premixed oils, at higher temperatures the reduction effect is increased attributed to the greater mobility of the additive palmitic acid. However, palmitic acid showed no effect on friction reduction for the DLC–DLC contacts at 25 °C and a slightly negative effect at 80 °C.204
2.9 Challenges of SAM for lubrication
The literature shows that the uniformity and hydrophobicity of SAMs are critical to friction reduction of the SAM coated silicon substrates. This has been shown above by head group selection and limited adsorption before tribological testing. Another factor is chain length, many of the studies mentioned have compared the difference and it is regularly reported that longer i.e. 18 carbons (OTS), performs best under a variety of tribological conditions. This appears to be valid over a range of substrates. This ability to reduce friction is commonly related to the ability to rearrange under shear stress with longer chains able to withstand compressive forces better. However, the durability of the SAMs is still a concern for practical applications as ultra-thin single SAMs easily break down under high loads or exhibit limited wear life. It has also been noted that high pressures and temperatures could remove monolayers.94 Fig. 15 shows that OTS can perform well under low loads in comparison to a bare silicon wafer (A and B), however under higher loads the monolayer will fail. That said, there is still a reduction in wear indicating that the OTS SAM did not fail instantaneously (C and D). | ||
| Fig. 15 Comparison of the wear tracks of a silicon wafer (A) compared to an OTS SAM (B) that has not failed. (D) Shows the wear track of an OTS SAM that has failed in comparison to a bare silicon wafer (C). (C and D) Reprinted with permission from ref. 70. (A and B) Reprinted with permission from ref. 162. | ||
Shear stress is a limiting factor of SAM lubrication, therefore research has focussed on forming composite SAM films or SAM multilayers. Multilayers, such as MAC, have shown promising results by improving the wear life of OTS SAM. These methods require different formation techniques such as extended spin coating or thermal annealing, for some applications such as in situ bearing lubrication this may not be feasible. This of course presents its own difficulties not just involving costs and maintenance but condition monitoring of lubricants. However, preformed SAM multilayers formed before use may prove feasible for applications. As there is no set testing standards the nature of investigations is inherently scattered. This obviously means that it is difficult to comprehend all the findings of a wide variety different contact size, geometries, load, speed etc.
3 Polymer brushes
Whilst SAMs are advantageous because of their simple formation, e.g. thiols on gold and chlorosilanes on oxides, and the end groups are easily modifiable for specific functions. Their application as a lubricant in tribological contacts is limited as thin film SAMs are susceptible to shearing.25,26 In a similar way, polymer brushes are formed by adsorption of prefabricated polymers onto the substrate through their reactive function groups or by subsequent polymerization onto reactive-terminal group containing SAMs. Polymer brushes typically have longer carbon chain and better durability than SAMs, thus better capabilities of polymer brushes to withstand compressive force and shear stress are expected for a better lubrication solution. Polymer brushes have been used to change wettability, reduce friction and increase corrosion resistance by using a variety of polymers.205–210 This section reviews the development of polymer brushes and their application in tribology systems.Polymer brushes are formed onto surfaces through two methods, named ‘grafting to’ and ‘grafting from’ processes. The ‘grafting to’ method uses prefabricated polymers that can be attached to a surface using physisorption or chemisorption much like SAM as seen in the left route of Fig. 16. The film thicknesses of polymers produced through the ‘grafting to’ method are limited by the molecular weights of the preformed polymer in solution.210,211 Although this method is relatively easy to carry out as it works much like a SAM, there is steric hindrance that impedes the density of the final film that is formed.71,212,213 In addition to this, the adsorption techniques are reversible so that the layers may be susceptible to high shear forces.210
 | ||
| Fig. 16 Schematics of the two different schemes of polymer brush formation and highlighting the density differences. The scheme also includes an AFM image showing (500 × 500 nm2) of poly(2-(methacryloyloxy)-ethyl-trimethyl-ammonium chloride) brushes grown from a Si wafer via surface initiated ATRP. Reprinted with permission from ref. 205. | ||
In the ‘grafting from’ techniques, also known as surface initiated polymerisations, the surface is first modified with a self-assembling initiator layer, which is then exposed to monomeric components with catalyst and, if needed, in an appropriate solvent.210,214–216 This can be seen in the right route of Fig. 16. It is imperative that the substrate to be modified has successfully had initiator molecules anchored to it, otherwise the solvent would become gelatinous due to polymerisation taking place. This in turn would then affect the density of the polymer film by way of steric hindrance.210 This method allows much more control over the final film and the grafting densities can approach 1 chain per nm2 (ref. 208, 211, 217–219) compared to the 0.05–0.1 chain per nm2 for ‘grafting to’ strategies.121,220 The improved density is due to the less steric hindrance from long chains to the substrate. In comparison to the limit of the films using ‘grafted to’ methods (<100 nm thickness), the ‘grafting from’ method can produce much thicker films.210
3.1 Radical polymerisation
There are many different polymerisation reactions that can be used to initiate polymer brushes, such as ring opening metathesis polymerisations,181,210,211,221 nitroxide mediated polymerisations,210,211,221,222 reversible addition fragmentation chain transfer polymerisation,181,210,211,221,222 atom transfer radical polymerisation,3,27,181,210–212,214,221–227 and living ring opening polymerisations.181,210,221 As ATRP is covered in depth its entirety is discussed in 3.2 Atom transfer radical polymerisation.3.2 Atom transfer radical polymerisation
ATRP is the most popular type of polymerisation for brushes due to the relative robustness of the technique. For example, unlike the other techniques, rigorously dry working conditions are not needed and reactions are very tolerant of a variety of monomers, ligands and catalysts. The commercial success of ATRP has been well reported, in part due to the polymerisations ability to be completed in commercially available equipment.210,213,221,227,238 ATRP can form well-defined polymer brushes from easily synthesised initiators and is well known as a successful controllable polymerisation.211ATRP was independently developed by Matyjaszewski and Sawamoto initially for controlled radical polymerisations239,240 and has been used to efficiently produce many different polymers223,224,241–243 due to its effective control in the composition and architecture of polymers. Surface initiated ATRP (SI-ATRP) was first reported by Huang et al. who used benzyl chloride attached to a trichlorosilane head to form a monolayer on silicon surface then poly acrylamide brushes using copper(I) chloride and bipyridine with acrylamide to create new stationary phases for chromatography.222,244 Copper is usually the transition metal choice for ATRP.27,121,222,241–243,245–247 Through ATRP, Ejaz et al. synthesised methyl methacrylate brushes on silicon wafers using a Cu based catalyst after a trimethoxysilane initiator was immobilised on the surface using Langmuir–Blodgett techniques.222,224,245 Since then the technique has been implemented onto gold, inorganic particles, organic latexes, formed dendrimers, highly functionalised linear brushes, and varied compositions and sizes.224
The basic structure for ATRP synthesis of polymer brushes is to have an initiator or monomer, and a catalyst made of a transition metal for successful grafting of chains. If the polymer brushes do not all grow at the same rate or time, the shorter chains can be inhibited from growing any further due to steric hindrance. This can be overcome by ensuring there are copious amounts of initiator sites on the substrate. There are two major steps in this polymerisation reaction, namely activation and deactivation. During the activation step, the metal complex breaks the alkyl halogen bond in the initiator, resulting in the formation of radicals. The radicals then propagate with the excess monomer and higher oxidation state metal complex. In the deactivation step, the radicals react with deactivators (e.g. polymer chain or activators) resulting in the formation of halide capped chains or reformed metal complex catalysts. For this to be successful, it is necessary to have a reversible reaction shifted to the dormant species, accompanied by fast initiation and deactivation. This is important as it can reduce the amount of termination steps.223,241 Due to the importance of ATRP in polymer brush development, it is further reviewed in the next section.
 | ||
| Scheme 1 Shows SI-ATRP, the attachment of the ATRP initiator (3-(2-bromoisobutyryl)propyl)dimethylchlorosilane to a silicon wafer. | ||
Liu and co-workers used the ATRP silane initiator (11′-trichlorosilylundecyl)-2-bromo-2-methylpropionate in their polymer brush formation.242 The silane initiator was synthesised using the esterification route detailed by Matyjaszewski,252 followed by hydrosilylation using trichlorosilane and Karstedt's catalyst as shown in Scheme 2. The authors also refer to the work of Husseman et al. for this synthesis. Husseman however, used Speier's catalyst instead.253 Karstedt's catalyst is more reactive requiring much lower amounts of Pt, Speier's catalyst is viewed as economically unattractive due to reactivity and that large amount of catalyst are rendered useless and unrecoverable.254 Bielecki et al. followed a very similar route except using chlorosilane which produced a less reactive head group as shown in Scheme 3.
 | ||
| Scheme 2 Shows the synthesis of the ATRP initiator (11′-trichlorosilylundecyl)-2-bromo-2-methylpropanoate via esterification and hydrosilylation. | ||
 | ||
| Scheme 3 Detailing the ATRP initiator (11′-chlorosilylundecyl)-2-bromo-2-methylpropanoate via esterification and hydrosilylation. | ||
Zhou et al. constructed an initiator monolayer on silica particles, then APTES self-assembled over the following 9 hours to produce an amide functionalised end group. This was then reacted with 2-bromoisobutyryl bromide (BIBB) and pyridine for 12 h to form the anchored initiator.255 Other initiators include 2-bromopropionyl bromide which reacts with the oxide layer and triethylamine (TEA) at 0 °C during gentle agitation.
| Transition metal | Transition metal complex ligand | Added ligand | Higher oxidation state transition metal (Y/N) | Temperature (°C) | Monomer | Reference |
|---|---|---|---|---|---|---|
| Cu | Br | PMDETA (N,N,N′,N′′,N′′-pentamethyldiethylenetriamine) | N | 90 | Lauryl acrylate | 261 |
| Cu | Cl | HMTETA (1,1,4,7,10,10-hexamethyltriethylene-tetraamine) | Y | 125 | Styrene methyl methacrylate | 242 |
| Cu | Br | 4,4′-Dinonyl-2,2′-dipyridyl | Y | 110 | Hexyl-, dodecyl- and octadecyl methacrylate | 27 |
| Cu | Br | 2,2′-Bipyridine | N | 90 | N-Isopropylacrylamide | 124 and 246 |
| Cu | Br | PMDETA | N | 40 | Oligo(ethylene glycol)methacrylate and 2-propynyl methacrylate | 243 |
| Cu | Br | 2,2′-Dipyridyl | N | RT | Methyl methacrylate | 267 |
| Cu | Br | PMDETA | N | RT | Benzylmethacrylate | 259 |
| Cu | Br | PMDETA | N | 90 | 2-(Dimethylamino)ethyl methacrylate | 255 |
| Cu | Br | Tris[2-(dimethylamino)ethyl]amine | Y | 30 | 4-Vinyl pyridine | 268 |
| Cu | Br | PMDETA | N | 50 | N-Isopropylacrylamide | 269 |
 | ||
| Scheme 5 Illustrating how the reducing agent can regenerate lost Cu(I) through oxidation or termination. Using information from ref. 211, 241, 258, 267, 270, 273 and 274. | ||
3.3 Polymer brushes synthesis
One of the main advantages of polymer brushes formed by ATRP is the controllability of the polymerisations.275 Similar to SAMs, polymer brushes are influenced by a range of factors such as temperature, solvent selection, presence of oxygen and free initiators. Unless otherwise mentioned, in this section polymer brushes are synthesised through self-assembling initiators and subsequent polymerisation from the active sites. An example is given in Scheme 6, showing a simplistic view of the polymerisation procedure from surface attached initiator monolayers to polymer growth. This was used by Munirasu et al. in forming polymer brushes of benzyl methacrylate with a copper catalyst, PMDETA ligand and free sacrificial initiator.259 | ||
| Fig. 18 Showing lauryl acrylate polymer brushes, some of which are partially collapsed and below a cross section. Reprinted with permission from ref. 261. | ||
Turan et al. studied the formation of poly(N-isopropylacrylamide) with the addition of a chain transfer agent 2-mercaptoethanol. Using varied amounts of 2-mercaptoethanol polymer brushes were formed at 90 °C over 18 h. The concentration of 2-mercaptoethanol was shown to control the chain length of the polymer brush with smaller concentrations dictating longer chain lengths. However, higher concentrations showed lower roughness although with reduced chain density.124 Turan et al. conducted another study on poly(N-isopropylacrylamide) polymer chains although without using an extra chemical additive to control polymerisation. Ellipsometry measurements showed a dry thickness of 66 nm. Munirasu et al. synthesised benzylmethacrylate polymer brushes as well as diblock copolymers with styrene on silicon wafer.259 Benzylmethacrylate polymer brushes followed a linear trend with time showing that brush length can be controlled with precision due to the additional EBIB. Their GPC results from the free polymer showed very low polydispersity indicating that the surface attached polymer grew at a uniform rate. Styrene performed similarly in respect to the EBIB-thickness trends. Liu and co-workers studied methyl methacrylate polymers, at 90 °C they varied the amount of a free sacrificial initiator. The thickest film produced had the lowest amount of EBIB added which is in agreement with Öztürk et al. Table 3 shows how the concentration of EBIB affects thickness, which is inherently correlated to Mn (number average molecular weight). Also notable is the slight decrease in polydispersity with minimal effect on contact angle.
The same experiments were done with solely styrene except at an elevated temperature by Liu et al.242 The data shows an irregular trend with 50 mM showing twice the thickness of the next highest thickness. The authors also created a random copolymer, using a ratio of methyl methacrylate to styrene of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and a range of EBIB concentrations the best compromise for conversion of monomers and thickness of film was found to be 50 mM however the thickest film was created by using 25 mMol L−1. When the ratios of monomers was inverted, the thickness was less predictable much like what was shown when the styrene was the only monomer limiting the influence of EBIB as a controlling sacrificial initiator. Zhou et al. constructed poly(2-(dimethylamino)ethylmethacrylate) brushes on silica particles, where a native oxide layer was found similar to that on silicon wafers and other silicon based substrates. Free polymer that was produced through the addition of EBIB was precipitated, this allowed gel permeation chromatography to take place. GPC results allow the calculation of Mn and molecular weight average (Mw) in addition to increasing the control of the reaction.255
1 and a range of EBIB concentrations the best compromise for conversion of monomers and thickness of film was found to be 50 mM however the thickest film was created by using 25 mMol L−1. When the ratios of monomers was inverted, the thickness was less predictable much like what was shown when the styrene was the only monomer limiting the influence of EBIB as a controlling sacrificial initiator. Zhou et al. constructed poly(2-(dimethylamino)ethylmethacrylate) brushes on silica particles, where a native oxide layer was found similar to that on silicon wafers and other silicon based substrates. Free polymer that was produced through the addition of EBIB was precipitated, this allowed gel permeation chromatography to take place. GPC results allow the calculation of Mn and molecular weight average (Mw) in addition to increasing the control of the reaction.255
When considering polymerisation time it is worth noting that at high levels of monomer conversion the rate of propagation will slow dramatically and end group functionality may be lost.28 Munirasu et al. synthesised benzylmethacrylate polymer brushes and achieved a thickness of above 300 nm after polymerisation for 37 hours as seen in Fig. 19.259 Fig. 19 also clearly shows a linear trend with time. Diblock polymers were synthesised to show that the end groups are still intact therefore polymerisation was not complete and end group functionality still remained.
 | ||
| Fig. 19 Showing the linear relationship between time and thickness of benzylmethacrylate polymer brushes. Reprinted with permission from ref. 259. | ||
Liu and co-workers synthesized polymer brushes from methyl methacrylate, styrene and a random copolymer consisting of both.242 When the authors studied methyl methacrylate as a standalone monomer, the conversions reached 99% at 90 °C with a small amount of EBIB. However, for styrene on its own, the conversion changed from 10% at 90 °C to 95% at 125 °C as can be seen in Fig. 20.242
 | ||
| Fig. 20 GPC results indicating low conversion of styrene at lower temperatures with increased polydispersity. Reprinted with permission from ref. 242. | ||
Song et al. produced a copolymer of oligo(ethylene glycol)methacrylate and 2-propynyl methacrylate.243 This was introduced in a 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 ratio to form the polymer brush. After 12 h at 40 °C the thickness of the brushes was found to be 26.8 nm. Interestingly, after formation of these films they were used for click chemistry, specifically click glycosylation.243 Zhou et al. formed poly(2-(dimethylamino)ethylmethacrylate) brushes over 12 h at 90 °C. Free polymer that was produced through the addition of free sacrificial initiator was precipitated, allowing gel permeation chromatography to take place and number average (Mn) was 6940 and molecular weight average (Mw) 11
40 ratio to form the polymer brush. After 12 h at 40 °C the thickness of the brushes was found to be 26.8 nm. Interestingly, after formation of these films they were used for click chemistry, specifically click glycosylation.243 Zhou et al. formed poly(2-(dimethylamino)ethylmethacrylate) brushes over 12 h at 90 °C. Free polymer that was produced through the addition of free sacrificial initiator was precipitated, allowing gel permeation chromatography to take place and number average (Mn) was 6940 and molecular weight average (Mw) 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870. Although polymerisation time was 4 hours less there is a considerable gap to the results produced by Liu et al. as can be seen in Table 3.255
870. Although polymerisation time was 4 hours less there is a considerable gap to the results produced by Liu et al. as can be seen in Table 3.255
3.4 Polymer brushes on silicon wafers for tribological applications
The tribological properties of polymer brushes are dependent on the surface attachment, the swelling behaviour of brushes if a solvent is present, the type of stress encountered as well as contact pressures.121 The pioneers of polymer brushes for tribology, Klein et al., also realised the importance of a good solvent to facilitate swelling and therefore better sliding performance.278 The immersion of polymer brushes in good solvent allows brush swelling, known to help lubricate as illustrated in Fig. 21.279–281 The mechanism in which solvated polymer brushes lubricate is thought to be as follows.121,282 | ||
| Fig. 21 An illustration of swollen polymer brushes lubricating a contact in comparison to poor solvent and a bare surface. Note the possible asperity contacts in poor solvent. | ||
(A) The resistance to rearrangement of the grafted chains with the repulsive nature of the brushes.
(B) Lubricant entrapment in the polymer brushes.
(C) High concentrations of lubricant in the outer polymer brush creates a low shear area protecting the brush system.
 | ||
| Fig. 22 Hexyl-, dodecyl- and octadecyl methacrylate polymer brushes formed from ATRP. Dry thicknesses measured by ellipsometry. Reprinted with permission from ref. 121. | ||
In a 20 cycle test with toluene all the brushes showed a low COF in the range of 0.01–0.02. It is worth noting that the much lower thickness in the P6MA polymer did not rule out a low COF in this case. The same experiment was completed using hexadecane, ethanol and PF350 oil as shown in Fig. 23. The improved reduction in friction when lubricated with non-polar liquids may be due to brush swelling improving the lubricating qualities as mentioned above. In this experiment the two longer chain and thicker polymer films faired considerably better than P6MA which increased the COF of friction. In all cases the lubricating qualities of the PF350 were higher than that of hexadecane. When the authors selected P12MA and created three different thicknesses of polymer, 70, 140 and 250 nm thick, the two lower coatings lost their friction reducing characteristics within a few meters. The 250 nm polymer film maintained a COF of 0.012 over more than 100 m.
 | ||
| Fig. 23 COF values for the polymer brushes in different lubricants or dry conditions. Reprinted with permission from ref. 27. | ||
The authors put this down to the fact that a thicker polymer film can be compressed more and reduce the likelihood of two hard counterparts coming into contact and the destructive high pressures destroying coatings. The application of a more viscous lubricant on the two thinner polymer coatings reduced the COF although the chemical instability may have been changed as there was a rapid failure of the 70 nm thick polymer in EO500 oil. The authors concluded that a greater initial brush thickness was more effective in separating the surfaces.27 Bhairamadgi et al. compared the adhesion and frictional characteristics of a polymer brush that was synthesised using fluorinated monomers to non-fluorinated monomers.284 The fluorinated polymer brush showed a strong correlation between thickness and adhesive pull off force in the tested range of 5–40 N. At every loading test brushes between 65–140 nm, thicker brushes resulted in a lower pull off force, between 9–29 nm there was some discrepancy but within error bars. Non fluorinated polymer brushes proved too high to measure. Lateral friction loading showed good correlation with other studies on polymer brush length vs. COF. Fluorinated polymer brushes of between 10–140 nm were analysed and the COF was reduced in an almost linear fashion throughout to a lowest friction of 0.0057. Similar to adhesion testing fluorinated polymers proved to be more successful at reducing friction than their non-fluorinated counterparts. The length of polymer brushes is also investigated by Sakata et al. who found that shorter chains of methyl methacrylate had a much larger margin of error.281 The authors also concluded a good quality solvent allows the extension of the brushes and in poor solvent they can be seen to be compressed. This was confirmed by tribological testing in good solvents, toluene and acetone, and bad solvents, hexane and cyclohexane. Similar behaviour has been observed with poly(2-methacryloyloxyethylphosphorylcholine) brushes and the swelling effects in poly(methylmethacrylate).285,286 However, these brushes are hydrophilic so water can provide a suitable aqueous lubricant. Nomura et al. varied the composition of solvent to subsequently effect the swelling of a polystyrene brush and therefore the lubricating qualities of the modification.262 Although not created by ATRP, Limpoco et al. produced 117.6 nm polystyrene brushes.287 The authors then varied solvent and Fig. 24 shows the effect. This data was collected through AFM measurements and shows that brush swelling not only effects macrotribology.
 | ||
| Fig. 24 AFM friction force vs. normal load in good and bad solvents. Reprinted with permission from ref. 287. | ||
Much like SAMs, friction is affected by the length of pre-formed adsorbed polymers. This is shown by Lin et al. who used the grafting-to approach.123 Three different copolymer brushes, poly(propylene oxide)–poly(ethylene oxide)–poly(propylene oxide), (PPO–PEO–PPO), were used as lubricants. Namely a PPO–PEO–PPOs with a molecular weights of 2700 with 60% PPO, 2150 with 80% PPO, 3100 with 80% PPO, designated as 17R4, 17R2 and 25R2 respectively. Tribological testing was carried out using a pin on disk tribometer. Ellipsometry measurements show that the thickest film produced was from the 25R4 polymer and reached around 6 nm, the thinnest film was 17R4 which reached nearly 1 nm. This again highlights the differences between grafting to and grafting from procedures. The better tribological performances were attributed to the longer chains and thicker films that had been formed.
 | ||
| Fig. 25 A representation of how polymer brushes can react with and without the presence of load. Reprinted with permission from ref. 269. | ||
The polymer brushes that Lin et al. previously introduced consistently reduce friction over the range of 0.01–0.1 m s−1.123 The effect of applied load was investigated by changing the load from 4–8 N under a constant sliding speed of 0.01 m s−1 and constant concentration. The notable changes in the COF are from 17R4 which increased between 4 and 5 N then levelled off, 25R2 shows a slight increase in COF throughout the entire test but until approximately 7.5 N surpassed the other polymers in friction reduction. 17R2 consistently showed COF values below 0.15 from 4–7 N where it sharply reduced. The COF fell below that of 15R2 after 7.5 N. Sun et al. sought to improve the tribological resistance of a polyamic acid polyimide film (PI).276 They achieved this by synthesizing polyglycidyl methacrylate brushes that are epoxy ended as an adhesive layer before application of the PI film. This both increased the lifetime of the film from 6000 sliding cycles to in excess of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 under a 0.5 N load at 20 mm s−1 with a consistently low COF of 0.08.
000 under a 0.5 N load at 20 mm s−1 with a consistently low COF of 0.08.
3.5 Polymer brushes on silicon nitride
Polymer brushes have been formed by ATRP on surfaces from silicon nitride cantilevers to wafers. However, very few tests have been done regarding tribology. de Groot et al. used a silicon nitride nanoporous substrate to form poly(methacrylic acid) brushes using ATRP.288 The ATRP was deposited via vapour phase deposition over 16 h. Sodium methacrylate was polymerised and in 1 h ellipsometry show that the film thickness was around 60 nm thick. The swell and collapse of the polymer brushes were also investigated using AFM. Nguyen et al. constructed a zwitterionic polymer brush on silicon nitride using ATRP.226 The silicon nitride was deposited upon silicon wafers using low pressure chemical vapour deposition, after etching with HF, 1,2-epoxy-9-decene was attached with UV light. After immersion in 1,2-ethlyamine the ATRP bromoisobutyryl bromide was attached using aforementioned techniques. The polymer brush of [3-(methacryloylamino)propyl]dimethyl(3-sulfopropyl)ammonium hydroxide inner salt monomers formed over 20 nm of growth in the first hour and 70 nm in 8 h. A later paper by Nguyen and co-workers detailed the same procedure for attachment of initiator and polymerisation upon a silicon nitride surface.289 The purpose of both polymer brushes created by Nguyen was protein repulsion. Gabriel et al. have created brushes on silicon nitride AFM probes, using electro initiated polymerisation techniques the authors successfully formed poly-N-succinimidylacrylate brushes.290 This was confirmed by approach and retraction curves using the cantilevers and a bare silicon substrate. The force curves could then be used as a form of sensing, much like monomers were used in CFM. Although not created by ATRP, Hartung et al. used a preformed copolymer brush like additive to lubricate Si3N4 contacts.180 Poly(L-lysine)-graft-poly(ethylene glycol) (PLL-g-PEG) was dissolved in a buffer solution of 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid in water (adjusted to pH 7), and samples were immersed for 30 minutes before testing was carried out. The cationic backbone adsorbs onto a negatively charged surface, hence the need for a buffer. Ellipsometry showed that the dry film thickness was approximately 15.7 nm. Tribological testing was completed using a pin on disk tribometer with a silicon nitride pin. Under a load of 5 N and speed of 120 mm s−1 the COF was 0.02 after running in under the buffer solution, after the addition of the buffer and polymer solution the COF dropped to 0.003. The solution also effectively lubricated under a slower speed of 10 mm s−1 under a load of 2 N which resulted in a COF of 0.04. However, the polymer inhibits the tribochemical reactions leading to a roughened surface and micro fractures.3.6 Challenges of polymer brushes for lubrication
As with SAM there is no standardised testing regime, subsequently this results in difficulties in comparing all polymer brushes. Previous studies show the importance of controlling polymer brushes, thicknesses, swelling behaviour, initiator attachment and catalyst systems, all of which and more must be considered when attempting polymer brush synthesis. As covered above the technology and ability to produce high quality polymer brushes is in existence. However, this will prove to be much more difficult when considering in situ lubrication, such as an additive like component in a lubricating fluid. This will be a main concern in macro systems, micro systems may well have similar issues. Another problem with in situ lubrication is the time required to produce the thicker brushes that have been shown to be capable of producing low COF. The stability of the Cu complex catalyst and the effect of the oxidative effects have been thoroughly documented, even with the developments of ARGET this will appear as a point of interest for the application of polymer brushes in years to come. It has also been found that the unstable copper catalyst could be reduced by iron and therefore lose its reactivity.27,291 Although this could be reversed by ARGET it becomes another complication of the polymer brush system. Although it has been shown that ATRP can be completed from silicon nitride and SAM attachment to silicon nitride has been proved, the lubrication of silicon nitride from SI-ATRP has not been completed yet.4 Outlook
This review has summarised the basics and the key development in SAMs and polymer brushes with a focus on tribology. Several decades have passed since the original study of self-assembling thiols on gold surfaces and SAMs have shown their feasibility for NEMS and MEMS lubrication. Although research has thrived in MEMS/NEMS, research has also been conducted to develop SAMs in macro applications especially on the controlling of interfaces and wettability that are key to the success of using SAMs. A multitude of SAMs have shown significant friction reducing characteristics which opens up possibilities of additive applications in automotive engines, certainly with ease of application in comparison to polymer brushes, however the shearing of such monolayers is still a concern. This growing area of research will continue to thrive due to the diverse disciplines that provide limitless potential applications.Following extensive research it is now possible to produce surface initiated polymer brushes upon surfaces with controlled polymerisations techniques. These living radical techniques such as ATRP have led to unrivalled control over chain length, architecture and composition. Polymer brushes are respected and established in polymer science and the interesting structures will lead to commercial opportunities. Limited research has been completed on the application of polymer brushes to silicon nitride especially with respect to lubrication. However, the ability to create thin polymer films with specific functions means that this challenge should be feasible. In addition, due to the structure of silicon nitride, lessons learned from silicon wafers and the expanding nanoparticle field should provide a bridging step. That said, the air sensitive nature of ATRP will be difficult to overcome to produce in situ polymerisations although developments such as ARGET could combat these problems. It should be remembered that polymer brushes for tribological applications encompasses a wide range of disciplines and all of the skills from these areas will be required to create the commercially viable solutions.
In summary, the authors think that the synthesis of polymer brushes through ATRP will meet the requirements of many tribological contacts. The wide range of monomers that can be polymerised, the robustness of the technique, coupled with developments such as ARGET are an attractive solution. Although we see polymer brushes as a solution for tribological contacts the very nature of surface initiation means the lessons learnt in SAMs must not be forgotten.
Acknowledgements
The authors acknowledge the financial support from the Defence Science and Technology Laboratory (DSTL, DSTLX1000093632), which is part of the UK's Ministry of Defence and from EPSRC (Grant EP/M50662X/1) to this project. The authors also thank D. Gropper for proofreading the manuscript.References
- K. Holmberg, P. Andersson and A. Erdemir, Tribol. Int., 2012, 47, 221–234 CrossRef PubMed.
- K. Holmberg, P. Andersson, N.-O. Nylund, K. Mäkelä and A. Erdemir, Tribol. Int., 2014, 78, 94–114 CrossRef PubMed.
- Z. Tang and S. Li, Curr. Opin. Solid State Mater. Sci., 2014, 18, 119–139 CrossRef CAS PubMed.
- M. Ratoi, V. B. Niste, H. Alghawel, Y. F. Suen and K. Nelson, RSC Adv., 2014, 4, 4278–4285 RSC.
- M. Ratoi, V. Anghel, C. Bovington and H. A. Spikes, Tribol. Int., 2000, 33, 241–247 CrossRef CAS.
- S. Loehlé, C. Matta, C. Minfray, T. L. Mogne, R. Iovine, Y. Obara, A. Miyamoto and J. M. Martin, Tribol. Int., 2015, 82(Part A), 218–227 CrossRef PubMed.
- V. Anghel, C. Bovington and H. A. Spikes, Lubr. Sci., 1999, 11, 313–335 CrossRef CAS PubMed.
- C. J. Reeves, P. L. Menezes, T.-C. Jen and M. R. Lovell, Tribol. Int., 2015, 90, 123–134 CrossRef CAS PubMed.
- H. Zhao, A. Neville, A. Morina, R. Vickerman and J. Durham, Tribol. Int., 2012, 46, 62–72 CrossRef CAS PubMed.
- T. Reddyhoff, I. S. Y. Ku, A. S. Holmes and H. A. Spikes, Tribol. Lett., 2011, 41, 239–246 CrossRef CAS.
- K. Topolovec Miklozic, J. Graham and H. Spikes, Tribol. Lett., 2001, 11, 71–81 CrossRef.
- A. Morina, A. Neville, M. Priest and J. H. Green, Tribol. Int., 2006, 39, 1545–1557 CrossRef CAS PubMed.
- S. Jiang, R. Frazier, E. S. Yamaguchi, M. Blanco, S. Dasgupta, Y. Zhou, T. Cagin, Y. Tang and W. A. Goddard, J. Phys. Chem. B, 1997, 101, 7702–7709 CrossRef CAS.
- W. Yue, C. Liu, Z. Fu, C. Wang, H. Huang and J. Liu, Mater. Des., 2014, 64, 601–607 CrossRef CAS PubMed.
- M. Skjoedt, R. Butts, D. N. Assanis and S. V. Bohac, Tribol. Int., 2008, 41, 556–563 CrossRef CAS PubMed.
- J. A. Williams, Tribol. Lett., 2004, 17, 765–778 CrossRef CAS.
- L. Wang, M. Nie and J. Rumbol, Tribol.-Mater., Surf. Interfaces, 2012, 6, 75–83 CrossRef CAS PubMed.
- S. Korcek, J. Sorab, M. D. Johnson and R. K. Jensen, Ind. Lubr. Tribol., 2000, 52, 209–220 CrossRef.
- H. Spikes, Lubr. Sci., 2008, 20, 103–136 CrossRef CAS PubMed.
- C. Haensch, S. Hoeppener and U. S. Schubert, Chem. Soc. Rev., 2010, 39, 2323–2334 RSC.
- C. Vericat, M. E. Vela, G. Benitez, P. Carro and R. C. Salvarezza, Chem. Soc. Rev., 2010, 39, 1805–1834 RSC.
- J. A. Williams and H. R. Le, J. Phys. D: Appl. Phys., 2006, 39, R201 CrossRef CAS.
- B. W. Ewers and J. D. Batteas, RSC Adv., 2014, 4, 16803–16812 RSC.
- R. L. Jones, B. L. Harrod and J. D. Batteas, Langmuir, 2010, 26, 16355–16361 CrossRef CAS PubMed.
- J. E. Raynor, J. R. Capadona, D. M. Collard, T. A. Petrie and A. J. García, Biointerphases, 2009, 4, FA3–FA16 CrossRef CAS PubMed.
- I. Luzinov, D. Julthongpiput, V. Gorbunov and V. V. Tsukruk, Tribol. Int., 2001, 34, 327–333 CrossRef CAS.
- R. Bielecki, M. Crobu and N. Spencer, Tribol. Lett., 2013, 49, 263–272 CrossRef CAS.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921–2990 CrossRef CAS PubMed.
- A. Ulman, Chem. Rev., 1996, 96, 1533–1554 CrossRef CAS PubMed.
- R. G. Nuzzo and D. L. Allara, J. Am. Chem. Soc., 1983, 105, 4481–4483 CrossRef CAS.
- S. A. Kulkarni, S. A. Mirji, A. B. Mandale, R. P. Gupta and K. P. Vijayamohanan, Mater. Lett., 2005, 59, 3890–3895 CrossRef CAS PubMed.
- T. Manifar, A. Rezaee, M. Sheikhzadeh and S. Mittler, Appl. Surf. Sci., 2008, 254, 4611–4619 CrossRef CAS PubMed.
- F. Schreiber, Prog. Surf. Sci., 2000, 65, 151–257 CrossRef CAS.
- S. Frank, J. Phys.: Condens. Matter, 2004, 16, R881 CrossRef.
- K. Bierbaum, M. Grunze, A. A. Baski, L. F. Chi, W. Schrepp and H. Fuchs, Langmuir, 1995, 11, 2143–2150 CrossRef CAS.
- D. K. Aswal, S. Lenfant, D. Guerin, J. V. Yakhmi and D. Vuillaume, Anal. Chim. Acta, 2006, 568, 84–108 CrossRef CAS PubMed.
- T. Balgar, R. Bautista, N. Hartmann and E. Hasselbrink, Surf. Sci., 2003, 532–535, 963–969 CrossRef CAS.
- R. Walsh, Acc. Chem. Res., 1981, 14, 246–252 CrossRef CAS.
- J. Dong, A. Wang, K. Y. S. Ng and G. Mao, Thin Solid Films, 2006, 515, 2116–2122 CrossRef CAS PubMed.
- K. Bierbaum, M. Kinzler, C. Woell, M. Grunze, G. Haehner, S. Heid and F. Effenberger, Langmuir, 1995, 11, 512–518 CrossRef CAS.
- T. A. Witten and L. M. Sander, Phys. Rev. Lett., 1981, 47, 1400–1403 CrossRef CAS.
- M. M. Sung, C. Carraro, O. W. Yauw, Y. Kim and R. Maboudian, J. Phys. Chem. B, 2000, 104, 1556–1559 CrossRef CAS.
- I. Doudevski, W. A. Hayes, J. T. Woodward and D. K. Schwartz, Colloids Surf., A, 2000, 174, 233–243 CrossRef CAS.
- D. Appelhans, D. Ferse, H. J. P. Adler, W. Plieth, A. Fikus, K. Grundke, F. J. Schmitt, T. Bayer and B. Adolphi, Colloids Surf., A, 2000, 161, 203–212 CrossRef CAS.
- D. K. Schwartz, S. Steinberg, J. Israelachvili and J. A. N. Zasadzinski, Phys. Rev. Lett., 1992, 69, 3354–3357 CrossRef CAS.
- Y. Wang and M. Lieberman, Langmuir, 2003, 19, 1159–1167 CrossRef CAS.
- M. Salmeron, Tribol. Lett., 2001, 10, 69–79 CrossRef CAS.
- I. Choi, Y. Kim, S. K. Kang, J. Lee and J. Yi, Langmuir, 2006, 22, 4885–4889 CrossRef CAS PubMed.
- I. Doudevski and D. K. Schwartz, J. Am. Chem. Soc., 2001, 123, 6867–6872 CrossRef CAS.
- V. V. Tsukruk, I. Luzinov and D. Julthongpiput, Langmuir, 1999, 15, 3029–3032 CrossRef CAS.
- J. J. Benítez, S. Kopta, D. F. Ogletree and M. Salmeron, Langmuir, 2002, 18, 6096–6100 CrossRef.
- Z. Kang, Q. Liu and Y. Liu, Wear, 2013, 303, 297–301 CrossRef CAS PubMed.
- N. Rozlosnik, M. C. Gerstenberg and N. B. Larsen, Langmuir, 2003, 19, 1182–1188 CrossRef CAS.
- D. K. Schwartz, Annu. Rev. Phys. Chem., 2001, 52, 107–137 CrossRef CAS PubMed.
- B. Cui, J. Zhang and J. Chen, Tribol. Int., 2013, 62, 149–154 CrossRef CAS PubMed.
- M. Masuko, H. Miyamoto and A. Suzuki, Tribol. Int., 2007, 40, 1587–1596 CrossRef CAS PubMed.
- N. Satyanarayana and K. S. Sujeet, J. Phys. D: Appl. Phys., 2005, 38, 3512 CrossRef CAS.
- S. Song, L. Liu and J. Zhang, Appl. Surf. Sci., 2011, 257, 10254–10260 CrossRef CAS PubMed.
- K. Wang, D. Xiong and Y. Niu, Appl. Surf. Sci., 2014, 317, 875–883 CrossRef CAS PubMed.
- D. Xiong, Y. Deng, N. Wang and Y. Yang, Appl. Surf. Sci., 2014, 298, 56–61 CrossRef CAS PubMed.
- L. G. Yu, E. S. Yamaguchi, M. Kasrai and G. M. Bancroft, Tribol. Int., 2011, 44, 692–701 CrossRef CAS PubMed.
- J. Zhao, M. Chen, Y. An, J. Liu and F. Yan, Appl. Surf. Sci., 2008, 255, 2295–2302 CrossRef CAS PubMed.
- B. D. Booth, N. J. Martin, E. A. Buehler, C. McCabe and G. K. Jennings, Colloids Surf., A, 2012, 412, 57–63 CrossRef CAS PubMed.
- K. Busuttil, N. Nikogeorgos, Z. Zhang, M. Geoghegan, C. A. Hunter and G. J. Leggett, Faraday Discuss., 2012, 156, 325–341 RSC.
- J. Feng, G. H. Xu, Y. An and X. Zeng, Colloids Surf., A, 2008, 316, 194–201 CrossRef CAS PubMed.
- N. R. Glass, R. Tjeung, P. Chan, L. Y. Yeo and J. R. Friend, Biomicrofluidics, 2011, 5, 36501–36507 CrossRef PubMed.
- D. S. Grierson and R. W. Carpick, Nano Today, 2007, 2, 12–21 CrossRef.
- J. Li and X. Z. Li, Appl. Surf. Sci., 2009, 255, 6159–6163 CrossRef CAS PubMed.
- S. Liu, J. Ou, Z. Li, S. Yang and J. Wang, Appl. Surf. Sci., 2012, 258, 2231–2236 CrossRef CAS PubMed.
- J. Q. Ma, C. J. Pang, Y. F. Mo and M. W. Bai, Wear, 2007, 263, 1000–1007 CrossRef CAS PubMed.
- M.-K. Park, G. Sakellariou, S. Pispas, N. Hadjichristidis and R. Advincula, Colloids Surf., A, 2008, 326, 115–121 CrossRef CAS PubMed.
- S.-L. Ren, S.-R. Yang, J.-Q. Wang, W.-M. Liu and Y.-P. Zhao, Chem. Mater., 2004, 16, 428–434 CrossRef CAS.
- C. Zhang, S. Zhang, P. Gao, H. Ma and Q. Wei, Thin Solid Films, 2014, 570(Part A), 27–32 CrossRef CAS PubMed.
- G. Yang, C. Zhang, S. Zhang, L. Yu and P. Zhang, Mater. Res. Bull., 2014, 55, 88–94 CrossRef CAS PubMed.
- N. Satyanarayana, S. K. Sinha and M. P. Srinivasan, in Tribology and Interface Engineering Series, ed. M. P. G. D. D. Dowson and A. A. Lubrecht, Elsevier, 2005, vol. 48, pp. 821–826 Search PubMed.
- S. Han, K. Suga, M. Fujihira and B.-E. Park, J. Korean Phys. Soc., 2014, 64, 63–68 CrossRef CAS.
- K. Raiber, A. Terfort, C. Benndorf, N. Krings and H.-H. Strehblow, Surf. Sci., 2005, 595, 56–63 CrossRef CAS PubMed.
- D. Skácelová, V. Danilov, J. Schäfer, A. Quade, P. Sťahel, M. Černák and J. Meichsner, J. Mater. Sci. Eng. B, 2013, 178, 651–655 CrossRef PubMed.
- D. Skácelová, M. Stupavská, P. Sťahel and M. Černák, Appl. Surf. Sci., 2014, 312, 203–207 CrossRef PubMed.
- L. Wu, L. Cai, A. Liu, W. Wang, Y. Yuan and Z. Li, Appl. Surf. Sci., 2015, 349, 683–694 CrossRef CAS PubMed.
- M. Wiegand, M. Reiche and U. Gösele, J. Electrochem. Soc., 2000, 147, 2734–2740 CrossRef CAS PubMed.
- V. Bhatt and S. Chandra, J. Electron. Mater., 2009, 38, 1979–1989 CrossRef CAS.
- R. Maboudian and C. Carraro, Annu. Rev. Phys. Chem., 2004, 55, 35–54 CrossRef CAS PubMed.
- D. Xu, W. H. Yu, E. T. Kang and K. G. Neoh, J. Colloid Interface Sci., 2004, 279, 78–87 CrossRef CAS PubMed.
- H. Sano, M. Zhao, D. Kasahara, K. Murase, T. Ichii and H. Sugimura, J. Colloid Interface Sci., 2011, 361, 259–269 CrossRef CAS PubMed.
- R. Boukherroub, Curr. Opin. Solid State Mater. Sci., 2005, 9, 66–72 CrossRef CAS PubMed.
- S. Y. Lee, Y. Choi, E. Ito, M. Hara, H. Lee and J. Noh, Phys. Chem. Chem. Phys., 2013, 15, 3609–3617 RSC.
- S. Hsieh, W.-J. Chao, P.-Y. Lin and C.-W. Hsieh, Corros. Sci., 2014, 80, 427–433 CrossRef CAS PubMed.
- M. E. McGovern, K. M. R. Kallury and M. Thompson, Langmuir, 1994, 10, 3607–3614 CrossRef CAS.
- L. T. Zhuravlev, Langmuir, 1987, 3, 316–318 CrossRef CAS.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103–1170 CrossRef CAS PubMed.
- A. Borruto, G. Crivellone and F. Marani, Wear, 1998, 222, 57–65 CrossRef CAS.
- B. D. Beake, J. S. G. Ling and G. J. Leggett, J. Mater. Chem., 1998, 8, 2845–2854 RSC.
- D. Devaprakasam, O. P. Khatri, N. Shankar and S. K. Biswas, Tribol. Int., 2005, 38, 1022–1034 CrossRef CAS PubMed.
- Z. Pawlak, W. Urbaniak and A. Oloyede, Wear, 2011, 271, 1745–1749 CrossRef CAS PubMed.
- R. Prioli, L. G. Jacobsohn, M. E. H. Maia da Costa and F. L. Freire Jr, Tribol. Lett., 2003, 15, 177–180 CrossRef CAS.
- Y. C. Jung and B. Bhushan, Langmuir, 2009, 25, 14165–14173 CrossRef CAS PubMed.
- E. Bormashenko, Y. Bormashenko, G. Whyman, R. Pogreb, A. Musin, R. Jager and Z. Barkay, Langmuir, 2008, 24, 4020–4025 CrossRef CAS PubMed.
- K.-Y. Yeh, L.-J. Chen and J.-Y. Chang, Langmuir, 2008, 24, 245–251 CrossRef CAS PubMed.
- M. Callewaert, C. Grandfils, L. Boulangé-Petermann and P. G. Rouxhet, J. Colloid Interface Sci., 2004, 276, 299–305 CrossRef CAS PubMed.
- D. Song, B. Song, H. Hu, X. Du and Z. Ma, Appl. Therm. Eng., 2015, 85, 356–364 CrossRef PubMed.
- S. Shibuichi, T. Yamamoto, T. Onda and K. Tsujii, J. Colloid Interface Sci., 1998, 208, 287–294 CrossRef CAS PubMed.
- D. Janssen, R. de Palma, S. Verlaak, P. Heremans and W. Dehaen, Thin Solid Films, 2006, 515, 1433–1438 CrossRef CAS PubMed.
- A. Cricenti, G. Longo, M. Luce, R. Generosi, P. Perfetti, D. Vobornik, G. Margaritondo, P. Thielen, J. S. Sanghera, I. D. Aggarwal, J. K. Miller, N. H. Tolk, D. W. Piston, F. Cattaruzza, A. Flamini, T. Prosperi and A. Mezzi, Surf. Sci., 2003, 544, 51–57 CrossRef CAS.
- H. Cheng and Y. Hu, Adv. Colloid Interface Sci., 2012, 171–172, 53–65 CrossRef CAS PubMed.
- H. Fischer, Macromolecules, 1997, 30, 5666–5672 CrossRef CAS.
- A. Checco, H. Schollmeyer, J. Daillant, P. Guenoun and R. Boukherroub, Langmuir, 2006, 22, 116–126 CrossRef CAS PubMed.
- J. Ma, J. Liu, Y. Mo and M. Bai, Colloids Surf., A, 2007, 301, 481–489 CrossRef CAS PubMed.
- B. Lego, M. François, W. G. Skene and S. Giasson, Langmuir, 2009, 25, 5313–5321 CrossRef CAS PubMed.
- C. Sun, M. Zhang, F. Zhou, P. Gao, Y. Xia and W. Liu, J. Macromol. Sci., Part B: Phys., 2011, 50, 1006–1017 CrossRef CAS PubMed.
- L. B. Picraux, C. D. Zangmeister and J. D. Batteas, Langmuir, 2006, 22, 174–180 CrossRef CAS PubMed.
- J. Tersoff and D. R. Hamann, Phys. Rev. Lett., 1983, 50, 1998–2001 CrossRef CAS.
- G. Binnig, H. Rohrer, C. Gerber and E. Weibel, Phys. Rev. Lett., 1982, 49, 57–61 CrossRef.
- G. Binnig and H. Rohrer, Rev. Mod. Phys., 1987, 59, 615–625 CrossRef CAS.
- M. Sprik, E. Delamarche, B. Michel, U. Roethlisberger, M. L. Klein, H. Wolf and H. Ringsdorf, Langmuir, 1994, 10, 4116–4130 CrossRef CAS.
- K. Edinger, A. Goelzhaeuser, K. Demota, C. Woell and M. Grunze, Langmuir, 1993, 9, 4–8 CrossRef CAS.
- L. Qin, W. Zhao, H. Hou, Y. Jin, Z. Zeng, X. Wu and Q. Xue, RSC Adv., 2014, 4, 60307–60315 RSC.
- S. V. Merzlikin, N. N. Tolkachev, T. Strunskus, G. Witte, T. Glogowski, C. Wöll and W. Grünert, Surf. Sci., 2008, 602, 755–767 CrossRef CAS PubMed.
- W. A. M. Aarnink, A. Weishaupt and A. van Silfhout, Appl. Surf. Sci., 1990, 45, 37–48 CrossRef CAS.
- A.-S. Duwez, J. Electron Spectrosc. Relat. Phenom., 2004, 134, 97–138 CrossRef CAS PubMed.
- R. Bielecki, E. Benetti, D. Kumar and N. Spencer, Tribol. Lett., 2012, 45, 477–487 CrossRef CAS.
- M. Biesalski and J. Rühe, Macromolecules, 2003, 36, 1222–1227 CrossRef CAS.
- B. Lin, A. K. Tieu, H. Zhu, B. Kosasih, O. Novareza and G. Triani, Wear, 2013, 302, 1010–1016 CrossRef CAS PubMed.
- E. Turan and T. Caykara, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 3880–3887 CrossRef CAS PubMed.
- J. J. Gooding, F. Mearns, W. Yang and J. Liu, Electroanalysis, 2003, 15, 81–96 CrossRef CAS PubMed.
- D. Samanta and A. Sarkar, Chem. Soc. Rev., 2011, 40, 2567–2592 RSC.
- A. Kumar, H. A. Biebuyck and G. M. Whitesides, Langmuir, 1994, 10, 1498–1511 CrossRef CAS.
- R. K. Smith, P. A. Lewis and P. S. Weiss, Prog. Surf. Sci., 2004, 75, 1–68 CrossRef CAS PubMed.
- M. Mrksich and G. M. Whitesides, Trends Biotechnol., 1995, 13, 228–235 CrossRef CAS.
- A. Y. Fadeev and T. J. McCarthy, Langmuir, 2000, 16, 7268–7274 CrossRef CAS.
- S.-J. Chun, S.-Y. Lee, G.-Y. Jeong and J. H. Kim, J. Ind. Eng. Chem., 2012, 18, 1122–1127 CrossRef CAS PubMed.
- B. D. Booth, S. G. Vilt, J. B. Lewis, J. L. Rivera, E. A. Buehler, C. McCabe and G. K. Jennings, Langmuir, 2011, 27, 5909–5917 CrossRef CAS PubMed.
- Y. Song, R. Premachandran Nair, M. Zou and Y. A. Wang, Thin Solid Films, 2010, 518, 3801–3807 CrossRef CAS PubMed.
- J. Cancino and S. A. S. Machado, Electrochim. Acta, 2012, 72, 108–113 CrossRef CAS PubMed.
- M. J. Wirth, R. W. P. Fairbank and H. O. Fatunmbi, Science, 1997, 275, 44–47 CrossRef CAS.
- J. Cancino, C. A. Razzino, V. Zucolotto and S. A. S. Machado, Electrochim. Acta, 2013, 87, 717–723 CrossRef CAS PubMed.
- T. Deng, J.-S. Li, S.-Y. Huan, H.-F. Yang, H. Wang, G.-L. Shen and R.-Q. Yu, Biosens. Bioelectron., 2006, 21, 1545–1552 CrossRef CAS PubMed.
- A. Brechling, M. Sundermann, U. Kleineberg and U. Heinzmann, Thin Solid Films, 2003, 433, 281–286 CrossRef CAS.
- M. V. Bracamonte, O. E. L. Pérez, M. L. Teijelo, G. A. Rivas and N. F. Ferreyra, Electrochim. Acta, 2014, 146, 178–185 CrossRef CAS PubMed.
- F. Bordi, C. Cametti and A. Gliozzi, Bioelectrochemistry, 2002, 57, 39–46 CrossRef CAS.
- H. T. Tien and A. L. Ottova, Colloids Surf., A, 1999, 149, 217–233 CrossRef CAS.
- R. P. Richter, R. Bérat and A. R. Brisson, Langmuir, 2006, 22, 3497–3505 CrossRef CAS PubMed.
- S. Terrettaz, M. Mayer and H. Vogel, Langmuir, 2003, 19, 5567–5569 CrossRef CAS.
- F. Berger, J. Delhalle and Z. Mekhalif, Appl. Surf. Sci., 2010, 256, 7131–7137 CrossRef CAS PubMed.
- Z. Zhu, G. Xu, Y. An and C. He, Colloids Surf., A, 2014, 457, 408–413 CrossRef CAS PubMed.
- K. Deng, R. J. Collins, M. Mehregany and C. N. Sukenik, J. Electrochem. Soc., 1995, 142, 1278–1285 CrossRef CAS PubMed.
- Y. Liu, P. Liu, Y. Xiao and J. Luo, Appl. Surf. Sci., 2012, 258, 8533–8537 CrossRef CAS PubMed.
- M. Cichomski, K. Kośla, J. Grobelny, W. Kozłowski and W. Szmaja, Appl. Surf. Sci., 2013, 273, 570–577 CrossRef CAS PubMed.
- L. Wang, R. J. K. Wood, T. J. Harvey, S. Morris, H. E. G. Powrie and I. Care, Wear, 2003, 255, 657–668 CrossRef CAS.
- F. E. Kennedy, Y. Lu and I. Baker, Tribol. Int., 2015, 82(Part B), 534–542 CrossRef CAS PubMed.
- R. Novak and T. Polcar, Tribol. Int., 2014, 74, 154–163 CrossRef CAS PubMed.
- D. L. Burris and W. G. Sawyer, Tribol. Lett., 2009, 35, 17–23 CrossRef.
- T. L. Schmitz, J. E. Action, J. C. Ziegert and W. G. Sawyer, J. Tribol., 2005, 127, 673–678 CrossRef.
- T. J. Kamps, J. C. Walker, R. J. Wood, P. M. Lee and A. G. Plint, Wear, 2015, 332–333, 1193–1199 CrossRef CAS PubMed.
- C. B. Mohan, C. Divakar, K. Venkatesh, K. Gopalakrishna, K. S. Mahesh Lohith and T. N. Naveen, Wear, 2009, 267, 1111–1116 CrossRef CAS PubMed.
- D. H. Hwang and K. H. Zum Gahr, Wear, 2003, 255, 365–375 CrossRef CAS.
- M. Kosinskiy, S. I.-U. Ahmed, Y. Liu and J. A. Schaefer, Tribol. Int., 2012, 56, 81–88 CrossRef PubMed.
- E.-S. Yoon, R. A. Singh, H.-J. Oh and H. Kong, Wear, 2005, 259, 1424–1431 CrossRef CAS PubMed.
- B. Bhushan and S. Sundararajan, Acta Mater., 1998, 46, 3793–3804 CrossRef CAS.
- S. P. Ho, R. W. Carpick, T. Boland and M. LaBerge, Wear, 2002, 253, 1145–1155 CrossRef CAS.
- B. Bhushan, Tribol. Int., 1995, 28, 85–96 CrossRef CAS.
- K.-H. Cha and D.-E. Kim, Wear, 2001, 251, 1169–1176 CrossRef.
- V. DePalma and N. Tillman, Langmuir, 1989, 5, 868–872 CrossRef CAS.
- E. E. Flater, W. R. Ashurst and R. W. Carpick, Langmuir, 2007, 23, 9242–9252 CrossRef CAS PubMed.
- M. Garcia-Parajo, C. Longo, J. Servat, P. Gorostiza and F. Sanz, Langmuir, 1997, 13, 2333–2339 CrossRef CAS.
- R. A. Singh, J. Kim, S. W. Yang, J.-E. Oh and E.-S. Yoon, Wear, 2008, 265, 42–48 CrossRef CAS PubMed.
- S. Strobl, T. Lube, P. Supancic, M. Stoiser, O. Schöppl and R. Danzer, J. Eur. Ceram. Soc., 2014, 34, 4167–4176 CrossRef CAS PubMed.
- L. Wang, R. W. Snidle and L. Gu, Wear, 2000, 246, 159–173 CrossRef CAS.
- K. Thoma, L. Rohr, H. Rehmann, S. Roos and J. Michler, Tribol. Int., 2004, 37, 463–471 CrossRef CAS PubMed.
- S. A. Baker, M. J. Dellavecchia, B. W. Smith and J. D. Winefordner, Anal. Chim. Acta, 1997, 355, 113–119 CrossRef CAS.
- R. C. Dante and C. K. Kajdas, Wear, 2012, 288, 27–38 CrossRef CAS PubMed.
- B. S. Bal and M. N. Rahaman, Acta Biomater., 2012, 8, 2889–2898 CrossRef CAS PubMed.
- V. Ferreira, H. N. Yoshimura and A. Sinatora, Wear, 2012, 296, 656–659 CrossRef CAS PubMed.
- Y. Hibi and Y. Enomoto, Wear, 1989, 133, 133–145 CrossRef CAS.
- J. Xu and K. Kato, Wear, 2000, 245, 61–75 CrossRef CAS.
- S. Hampshire, Journal of Achievements of Materials and Manufacturing Engineering, 2007, 24, 43–50 Search PubMed.
- F. L. Riley, J. Am. Ceram. Soc., 2000, 83, 245–265 CrossRef CAS PubMed.
- H. Bhaskaran, B. Gotsmann, A. Sebastian, U. Drechsler, M. A. Lantz, M. Despont, P. Jaroenapibal, R. W. Carpick, Y. Chen and K. Sridharan, Nat. Nanotechnol., 2010, 5, 181–185 CrossRef CAS PubMed.
- R. S. Gates and S. M. Hsu, Tribol. Trans., 2000, 43, 269–274 CrossRef CAS PubMed.
- W. Hartung, A. Rossi, S. Lee and N. Spencer, Tribol. Lett., 2009, 34, 201–210 CrossRef CAS.
- H. K. Hunt and A. M. Armani, IEEE J. Sel. Top. Quantum Electron., 2014, 20, 121–133 CrossRef.
- M. M. Sung, G. J. Kluth and R. Maboudian, J. Vac. Sci. Technol., A, 1999, 17, 540–544 CAS.
- G. J. Kluth, M. M. Sung and R. Maboudian, Langmuir, 1997, 13, 3775–3780 CrossRef CAS.
- M. Kölbel, R. W. Tjerkstra, G. Kim, J. Brugger, C. J. M. van Rijn, W. Nijdam, J. Huskens and D. N. Reinhoudt, Adv. Funct. Mater., 2003, 13, 219–224 CrossRef PubMed.
- J. Diao, D. Ren, J. R. Engstrom and K. H. Lee, Anal. Biochem., 2005, 343, 322–328 CrossRef CAS PubMed.
- R. Barattin and N. Voyer, Chem. Commun., 2008, 1513–1532 RSC.
- V. V. Tsukruk and V. N. Bliznyuk, Langmuir, 1998, 14, 446–455 CrossRef CAS.
- B. Daniel and H. Christofer, J. Micromech. Microeng., 2007, 17, 1326 CrossRef.
- U. Zaghloul, G. Papaioannou, B. Bhushan, F. Coccetti, P. Pons and R. Plana, Microelectron. Reliab., 2011, 51, 1810–1818 CrossRef CAS PubMed.
- Z. Yapu, Acta Mech. Sin., 2003, 19, 1–10 CrossRef.
- E. Kutnyanszky and G. J. Vancso, Eur. Polym. J., 2012, 48, 8–15 CrossRef CAS PubMed.
- T. Ito, M. Namba, P. Bühlmann and Y. Umezawa, Langmuir, 1997, 13, 4323–4332 CrossRef CAS.
- J. E. Headrick and C. L. Berrie, Langmuir, 2004, 20, 4124–4131 CrossRef CAS.
- C. D. Frisbie, L. F. Rozsnyai, A. Noy, M. S. Wrighton and C. M. Lieber, Science, 1994, 265, 2071–2074 CAS.
- S. S. Kelkar, D. Chiavetta and C. A. Wolden, Appl. Surf. Sci., 2013, 282, 291–296 CrossRef CAS PubMed.
- D. Wang, Y. Ni, Q. Huo and D. E. Tallman, Thin Solid Films, 2005, 471, 177–185 CrossRef CAS PubMed.
- B. Panjwani and S. K. Sinha, J. Mech. Behav. Biomed. Mater., 2012, 15, 103–111 CrossRef CAS PubMed.
- P. F. Li, Y. Xu and X.-H. Cheng, Surf. Coat. Technol., 2013, 232, 331–339 CrossRef CAS PubMed.
- P. F. Li, H. Zhou and X.-H. Cheng, Appl. Surf. Sci., 2013, 285(Part B), 937–944 CrossRef CAS PubMed.
- S. Jadhav, Cent. Eur. J. Chem., 2011, 9, 369–378 CrossRef CAS.
- M. Ratoi, C. Bovington and H. Spikes, J. Jpn. Soc. Tribol., 2001, 1281–1286 Search PubMed.
- Y. Wan, Y. Wang, Q. Zhang, Z. Wang, Z. Xu, C. Liu and J. Zhang, Appl. Surf. Sci., 2012, 259, 147–152 CrossRef CAS PubMed.
- Q. Zhang, Y. Wan, Y. Li, S. Yang and W. Yao, Appl. Surf. Sci., 2013, 280, 545–549 CrossRef CAS PubMed.
- R. Simič and M. Kalin, Appl. Surf. Sci., 2013, 283, 460–470 CrossRef PubMed.
- O. Azzaroni, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3225–3258 CrossRef CAS PubMed.
- Q. Wei, M. Cai, F. Zhou and W. Liu, Macromolecules, 2013, 46, 9368–9379 CrossRef CAS.
- J. Yan, B. Li, F. Zhou and W. Liu, ACS Macro Lett., 2013, 2, 592–596 CrossRef CAS.
- R. Bielecki, P. Doll and N. Spencer, Tribol. Lett., 2013, 49, 273–280 CrossRef CAS.
- T. Goren, N. D. Spencer and R. Crockett, RSC Adv., 2014, 4, 21497–21503 RSC.
- S. Edmondson, V. L. Osborne and W. T. S. Huck, Chem. Soc. Rev., 2004, 33, 14–22 RSC.
- B. Li, B. Yu, Q. Ye and F. Zhou, Acc. Chem. Res., 2015, 48, 229–237 CrossRef CAS PubMed.
- S. Edmondson and S. P. Armes, Polym. Int., 2009, 58, 307–316 CrossRef CAS PubMed.
- S. Edmondson, C.-D. Vo, S. P. Armes and G.-F. Unali, Macromolecules, 2007, 40, 5271–5278 CrossRef CAS.
- S. Peng and B. Bhushan, RSC Adv., 2012, 2, 8557–8578 RSC.
- F. Zhou and W. T. S. Huck, Phys. Chem. Chem. Phys., 2006, 8, 3815–3823 RSC.
- C. Xu, T. Wu, J. D. Batteas, C. M. Drain, K. L. Beers and M. J. Fasolka, Appl. Surf. Sci., 2006, 252, 2529–2534 CrossRef CAS PubMed.
- C. Yoshikawa, J. Qiu, C.-F. Huang, Y. Shimizu, J. Suzuki and E. van den Bosch, Colloids Surf., B, 2015, 127, 213–220 CrossRef CAS PubMed.
- Y. Inoue and K. Ishihara, Colloids Surf., B, 2010, 81, 350–357 CrossRef CAS PubMed.
- B. F. L. Lai, A. L. Creagh, J. Janzen, C. A. Haynes, D. E. Brooks and J. N. Kizhakkedathu, Biomaterials, 2010, 31, 6710–6718 CrossRef CAS PubMed.
- S. Yamamoto, M. Ejaz, Y. Tsujii and T. Fukuda, Macromolecules, 2000, 33, 5608–5612 CrossRef CAS.
- A. Olivier, F. Meyer, J.-M. Raquez, P. Damman and P. Dubois, Prog. Polym. Sci., 2012, 37, 157–181 CrossRef CAS PubMed.
- R. Barbey, L. Lavanant, D. Paripovic, N. Schüwer, C. Sugnaux, S. Tugulu and H.-A. Klok, Chem. Rev., 2009, 109, 5437–5527 CrossRef CAS PubMed.
- W. Hadasha and B. Klumperman, Polym. Int., 2014, 63, 824–834 CrossRef CAS PubMed.
- J. Pyun, T. Kowalewski and K. Matyjaszewski, Macromol. Rapid Commun., 2003, 24, 1043–1059 CrossRef CAS PubMed.
- R. Toomey and M. Tirrell, Annu. Rev. Phys. Chem., 2008, 59, 493–517 CrossRef CAS PubMed.
- A. T. Nguyen, J. Baggerman, J. M. J. Paulusse, C. J. M. van Rijn and H. Zuilhof, Langmuir, 2011, 27, 2587–2594 CrossRef CAS PubMed.
- C. Kang, R. M. Crockett and N. D. Spencer, Macromolecules, 2014, 47, 269–275 CrossRef CAS.
- O. Nuyken and S. Pask, Polymer, 2013, 5, 361 Search PubMed.
- S. Penczek and G. Moad, Pure Appl. Chem., 2008, vol. 80, 2163 CrossRef.
- K. Udipi, R. S. Davé, R. L. Kruse and L. R. Stebbins, Polymer, 1997, 38, 927–938 CrossRef CAS.
- C. W. Bielawski and R. H. Grubbs, Prog. Polym. Sci., 2007, 32, 1–29 CrossRef CAS PubMed.
- C. J. Hawker, J. Am. Chem. Soc., 1994, 116, 11185–11186 CrossRef CAS.
- D. Benoit, V. Chaplinski, R. Braslau and C. J. Hawker, J. Am. Chem. Soc., 1999, 121, 3904–3920 CrossRef CAS.
- M. Semsarilar and S. Perrier, Nat. Chem., 2010, 2, 811–820 CrossRef CAS PubMed.
- J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 1998, 31, 5559–5562 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2005, 58, 379–410 CrossRef CAS.
- B. Giechaskiel, M. Maricq, L. Ntziachristos, C. Dardiotis, X. Wang, H. Axmann, A. Bergmann and W. Schindler, J. Aerosol Sci., 2014, 67, 48–86 CrossRef CAS PubMed.
- J. Yan, B. Li, B. Yu, W. T. S. Huck, W. Liu and F. Zhou, Angew. Chem., Int. Ed., 2013, 52, 9125–9129 CrossRef CAS PubMed.
- M. Kato, M. Kamigaito, M. Sawamoto and T. Higashimura, Macromolecules, 1995, 28, 1721–1723 CrossRef CAS.
- J.-S. Wang and K. Matyjaszewski, J. Am. Chem. Soc., 1995, 117, 5614–5615 CrossRef CAS.
- P. Król and P. Chmielarz, Prog. Org. Coat., 2014, 77, 913–948 CrossRef PubMed.
- H. Liu, C. T. O'Mahony, F. Audouin, C. Ventura, M. Morris and A. Heise, Macromol. Chem. Phys., 2012, 213, 108–115 CrossRef CAS PubMed.
- W. Song, C. Xiao, L. Cui, Z. Tang, X. Zhuang and X. Chen, Colloids Surf., B, 2012, 93, 188–194 CrossRef CAS PubMed.
- X. Huang and M. J. Wirth, Anal. Chem., 1997, 69, 4577–4580 CrossRef CAS.
- M. Ejaz, S. Yamamoto, K. Ohno, Y. Tsujii and T. Fukuda, Macromolecules, 1998, 31, 5934–5936 CrossRef CAS.
- E. Turan, S. Demirci and T. Caykara, Thin Solid Films, 2010, 518, 5950–5954 CrossRef CAS PubMed.
- H. Fischer, Chem. Rev., 2001, 101, 3581–3610 CrossRef CAS PubMed.
- H. Gao and K. Matyjaszewski, Prog. Polym. Sci., 2009, 34, 317–350 CrossRef CAS PubMed.
- K. Matyjaszewski, Macromolecules, 2012, 45, 4015–4039 CrossRef CAS.
- L. J. T. Landherr, C. Cohen, P. Agarwal and L. A. Archer, Langmuir, 2011, 27, 9387–9395 CrossRef CAS PubMed.
- K. Ohno, T. Morinaga, K. Koh, Y. Tsujii and T. Fukuda, Macromolecules, 2005, 38, 2137–2142 CrossRef CAS.
- K. Matyjaszewski, P. J. Miller, N. Shukla, B. Immaraporn, A. Gelman, B. B. Luokala, T. M. Siclovan, G. Kickelbick, T. Vallant, H. Hoffmann and T. Pakula, Macromolecules, 1999, 32, 8716–8724 CrossRef CAS.
- M. Husseman, E. E. Malmström, M. McNamara, M. Mate, D. Mecerreyes, D. G. Benoit, J. L. Hedrick, P. Mansky, E. Huang, T. P. Russell and C. J. Hawker, Macromolecules, 1999, 32, 1424–1431 CrossRef CAS.
- B. D. Karstedt, US Pat., US3775452, 1971.
- W. Zhou, H. Liu, H. Ye, H. Cui, R. Wang, J. Li and X. Zhang, Powder Technol., 2013, 249, 1–6 CrossRef CAS PubMed.
- W. Feng, J. L. Brash and S. Zhu, Biomaterials, 2006, 27, 847–855 CrossRef CAS PubMed.
- D. J. Siegwart, J. K. Oh and K. Matyjaszewski, Prog. Polym. Sci., 2012, 37, 18–37 CrossRef CAS PubMed.
- K. Matyjaszewski, H. Dong, W. Jakubowski, J. Pietrasik and A. Kusumo, Langmuir, 2007, 23, 4528–4531 CrossRef CAS PubMed.
- S. Munirasu, R. G. Karunakaran, J. Rühe and R. Dhamodharan, Langmuir, 2011, 27, 13284–13292 CrossRef CAS PubMed.
- E. Turan and T. Caykara, React. Funct. Polym., 2011, 71, 1089–1095 CrossRef CAS PubMed.
- E. Öztürk, E. Turan and T. Caykara, Appl. Surf. Sci., 2010, 257, 1015–1020 CrossRef PubMed.
- A. Nomura, K. Okayasu, K. Ohno, T. Fukuda and Y. Tsujii, Macromolecules, 2011, 44, 5013–5019 CrossRef CAS.
- S. Yamamoto, M. Ejaz, Y. Tsujii, M. Matsumoto and T. Fukuda, Macromolecules, 2000, 33, 5602–5607 CrossRef CAS.
- D. A. Shipp and K. Matyjaszewski, Macromolecules, 2000, 33, 1553–1559 CrossRef CAS.
- K. L. Beers, S. G. Gaynor, K. Matyjaszewski, S. S. Sheiko and M. Möller, Macromolecules, 1998, 31, 9413–9415 CrossRef CAS.
- T. Pintauer and K. Matyjaszewski, Coord. Chem. Rev., 2005, 249, 1155–1184 CrossRef CAS PubMed.
- B. Zhu and S. Edmondson, Polymer, 2011, 52, 2141–2149 CrossRef CAS PubMed.
- F. Jiang, W. H. Meyer and J. Zhang, Colloids Surf., A, 2013, 436, 302–308 CrossRef CAS PubMed.
- Y. Liu, Y. Xiao and J. Luo, Sci. China: Technol. Sci., 2012, 55, 3352–3358 CrossRef CAS.
- A. M. Elsen, J. Burdyńska, S. Park and K. Matyjaszewski, ACS Macro Lett., 2013, 2, 822–825 CrossRef CAS.
- J. Ran, L. Wu, Z. Zhang and T. Xu, Prog. Polym. Sci., 2014, 39, 124–144 CrossRef CAS PubMed.
- N. V. Tsarevsky and K. Matyjaszewski, Chem. Rev., 2007, 107, 2270–2299 CrossRef CAS PubMed.
- K. Min, H. Gao and K. Matyjaszewski, Macromolecules, 2007, 40, 1789–1791 CrossRef CAS.
- W. Jakubowski and K. Matyjaszewski, Macromolecules, 2005, 38, 4139–4146 CrossRef CAS.
- T. Pintauer and K. Matyjaszewski, Chem. Soc. Rev., 2008, 37, 1087–1097 RSC.
- C. Sun, F. Zhou, L. Shi, B. Yu, P. Gao, J. Zhang and W. Liu, Appl. Surf. Sci., 2006, 253, 1729–1735 CrossRef CAS PubMed.
- F. Seeliger and K. Matyjaszewski, Macromolecules, 2009, 42, 6050–6055 CrossRef CAS.
- J. Klein, E. Kumacheva, D. Mahalu, D. Perahia and L. J. Fetters, Nature, 1994, 370, 634–636 CrossRef CAS PubMed.
- G. Fontani, R. Gaspari, N. D. Spencer, D. Passerone and R. Crockett, Langmuir, 2013, 29, 4760–4771 CrossRef CAS PubMed.
- R. Heeb, R. M. Bielecki, S. Lee and N. D. Spencer, Macromolecules, 2009, 42, 9124–9132 CrossRef CAS.
- H. Sakata, M. Kobayashi, H. Otsuka and A. Takahara, Polym. J., 2005, 37, 767–775 CrossRef CAS.
- D. Irfachsyad, D. Tildesley and P. Malfreyt, Phys. Chem. Chem. Phys., 2002, 4, 3008–3015 RSC.
- O. Tairy, N. Kampf, M. J. Driver, S. P. Armes and J. Klein, Macromolecules, 2015, 48, 140–151 CrossRef CAS.
- N. S. Bhairamadgi, S. P. Pujari, F. A. M. Leermakers, C. J. M. van Rijn and H. Zuilhof, Langmuir, 2014, 30, 2068–2076 CrossRef CAS PubMed.
- M. Kobayashi, Y. Terayama, N. Hosaka, M. Kaido, A. Suzuki, N. Yamada, N. Torikai, K. Ishihara and A. Takahara, Soft Matter, 2007, 3, 740–746 RSC.
- L. C. H. Moh, M. D. Losego and P. V. Braun, Langmuir, 2011, 27, 3698–3702 CrossRef CAS PubMed.
- F. T. Limpoco, R. C. Advincula and S. S. Perry, Langmuir, 2007, 23, 12196–12201 CrossRef CAS PubMed.
- G. W. de Groot, M. G. Santonicola, K. Sugihara, T. Zambelli, E. Reimhult, J. Vörös and G. J. Vancso, ACS Appl. Mater. Interfaces, 2013, 5, 1400–1407 CAS.
- A. T. Nguyen, J. Baggerman, J. M. J. Paulusse, H. Zuilhof and C. J. M. van Rijn, Langmuir, 2012, 28, 604–610 CrossRef CAS PubMed.
- S. Gabriel, C. Jérôme, R. Jérôme, C.-A. Fustin, A. Pallandre, J. Plain, A. M. Jonas and A.-S. Duwez, J. Am. Chem. Soc., 2007, 129, 8410–8411 CrossRef CAS PubMed.
- R. Gong, S. Maclaughlin and S. Zhu, Appl. Surf. Sci., 2008, 254, 6802–6809 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |