Highly efficient dehydrogenative cross-coupling of aldehydes with amines and alcohols†
Abstract
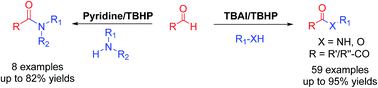
A common protocol for the synthesis of amides, esters and α-ketoesters via cross dehydrogenative coupling of aldehydes and amines/alcohols has been developed. The method is applicable to a wide variety of alcohols and amines as well as aliphatic and aromatic aldehydes. Also, the use of acetaldehyde for acetylation and ethyl glyoxalate to access 2-oxo-amino esters is presented for the first time.

 Please wait while we load your content...
Please wait while we load your content...