Friedel–Crafts reaction of indoles with vicinal tricarbonyl compounds generated in situ from 1,3-dicarbonyl compounds and TEMPO: highly selective synthesis of tertiary alcohols†
Jianwei Yan*a,
Tianjun Nib,
Fulin Yana,
Jixia Zhanga and
Fangfang Zhuanga
aSchool of Pharmacy, Xinxiang Medical University, Xinxiang, Henan 453003, P. R. China. E-mail: jwyansimm@163.com
bDepartment of Chemistry, Xinxiang Medical University, Xinxiang, Henan 453003, P. R. China
First published on 15th October 2015
Abstract
A novel Friedel–Crafts reaction of indoles with vicinal tricarbonyl compounds generated in situ from 1,3-dicarbonyl compounds, which produces indole substituted tertiary alcohols in good to excellent yields and with good functional group tolerance, has been developed. The mechanistic pathway for this process involves the initial disproportionation of TEMPO, α-oxyamination of the 1,3-dicarbonyl compound followed by N–O bond cleavage to form the tricarbonyl intermediate. Addition of indole to this intermediate then generates the tertiary alcohol product. This method can also be used for the synthesis of pyrrole-containing tertiary alcohols. Further efforts aimed at elucidating the mechanism of the reaction and boardening the substrate scope of this process are ongoing.
Introduction
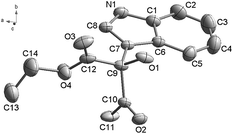
The indole ring system is the core structure of over 3000 natural products and dozens of pharmaceutical agents.1 Owing to their ubiquitous nature, functionalized indoles have been the focus of numerous synthetic studies. A large effort has been given recently to the development of new indole C–H activation processes that can be used to prepare indole derivatives.2 Over the past several decades, great advances have been made in devising transition metal-catalyzed C(2)–H or C(3)–H functionalization reactions of indoles3 owing to their broad functional group tolerance, these processes have played significant roles in the synthesis and modification of indole containing medicinal agents. Direct double C–H functionalization reactions between indoles and substrates containing C(sp3)–H bonds, leading to formation of C(sp2)–C(sp3) bonds have potent application in synthetic organic chemistry owing to unique advantages associated with simplicity and the accessibilities of starting materials. Numerous efforts conducted to develop processes of this type have resulted in new preparative methods involving cross-dehydrogenative coupling of indoles with tetrahydroisoquinolines4 and tertiary amines,5 and reactions of indoles with methyl ketones,6 α-amino carbonyl compounds,7 and secondary and tertiary amine.8 Recently, Pihko and co-workers described a palladium-catalyzed dehydrogenative β′-functionalization reaction of cyclic β-ketoesters with indoles9 and Gribble and co-workers devised a Mn(III)-mediated radical addition reaction of 2-nitroindole and 2-cyanoindole with activated methylene compounds such as malonates.10The 2,2,6,6-tetramethylpiperidine-N-oxyl radical (TEMPO), has been broadly used as a stoichiometric and catalytic oxidant in organic reactions.11 In the past several years, TEMPO also been used as an oxidant in novel, transition metal-catalyzed, C–C bond forming reactions12 and the α-C–H activation of carbonyl compounds.13 In addition, TEMPO was also applied in the α-oxygenation of 1,3-dicarbonyl compounds.14 We envisaged that TEMPO would promote the formation of C–C bonds between the C-3 position of indoles and the α-position of 1,3-dicarbonyl compounds, to form novel molecular scaffold. In order to test this proposal, a mixture of indole (1a), ethyl acetoacetate (2a) (1.1 equiv.), and TEMPO (2.0 equiv.) in acetic acid was stirred at ambient temperatures open to air for 30 h (eqn (1)). Under these conditions, efficient (81%) reaction took place to generate the tertiary alcohol 3aa, whose structure was unambiguously assigned using X-ray crystallographic analysis (Fig. 1).15
To date, the preparation method of indole-containing tertiary alcohol mainly through the Friedel–Crafts reaction of indoles with ethyl trifluoropyruvate,16 2-oxomalonate17 and other α-ketoesters.18 The general method for the synthesis of indole-containing is still needed. Below, we describe the results a thorough study of this one-pot process for the preparation of indole-containing tertiary alcohol.
Results and discussion
In the first phase of this effort, studies were carried out to determine optimal conditions for the tertiary alcohol forming reaction between indole and ethyl acetoacetate. Increasing the temperature to 50 °C enabled the alcohol forming reaction to be completed in 8 h (Table 1, entry 3), whereas further increasing the temperature to 80 °C led to a obviously reduced yield (Table 1, entry 5). Importantly, when the amount of TEMPO was increased from 2.0 to 3.0 equivalents, the reaction generated 3aa in a 85% yield (Table 1, entry 3), while decreasing the amount of TEMPO to 1.1 equivalents resulted in a significantly decreased yield (Table 1, entry 4). The results of screening carboxylic acid solvents showed that the domino process is sensitive to the acidity of the medium. Specifically, reaction in formic acid was observed to produce a complicated product mixture (Table 1, entry 6), whereas when acetic (optimal), propionic, butyric and valeric acid are used as solvents the tandem process proceeded efficiently (Table 1, entries 7–9). In addition, reaction conducted under an argon atmosphere took place with a decreased yield (Table 1, entry 10). The combined results show that optimal conditions for reaction of indole (1 equiv.) with ethyl acetoacetate (1.1 equiv.) involve the use of 3.0 equivalents TEMPO, acetic acid as solvent, a temperature of 50 °C and an exposure to air for 1 h.| Entry | Solvent | TEMPO | Temp/time | Yieldb |
|---|---|---|---|---|
| a Reaction conditions: a mixture of indole 1a (1.0 mmol), ethyl acetoacetate 2a (1.1 mmol) and TEMPO in the designated solvent was stirred at the indicated temperatures for the times indicated open to air.b Isolated yields.c Under Ar. | ||||
| 1 | MeCO2H | 2.0 equiv. | r.t./30 h | 81% |
| 2 | MeCO2H | 2.0 equiv. | 50 °C/8 h | 78% |
| 3 | MeCO2H | 3.0 equiv. | 50 °C/1 h | 85% |
| 4 | MeCO2H | 1.1 equiv. | 50 °C/48 h | 52% |
| 5 | MeCO2H | 3.0 equiv. | 80 °C/0.5 h | 71% |
| 6 | HCO2H | 3.0 equiv. | r.t./0.5 h | Mess |
| 7 | EtCO2H | 3.0 equiv. | 50 °C/8 h | 81% |
| 8 | PrCO2H | 3.0 equiv. | 50 °C/16 h | 69% |
| 9 | BuCO2H | 3.0 equiv. | 50 °C/24 h | 69% |
| 10c | MeCO2H | 3.0 equiv. | 50 °C/1 h | 76% |
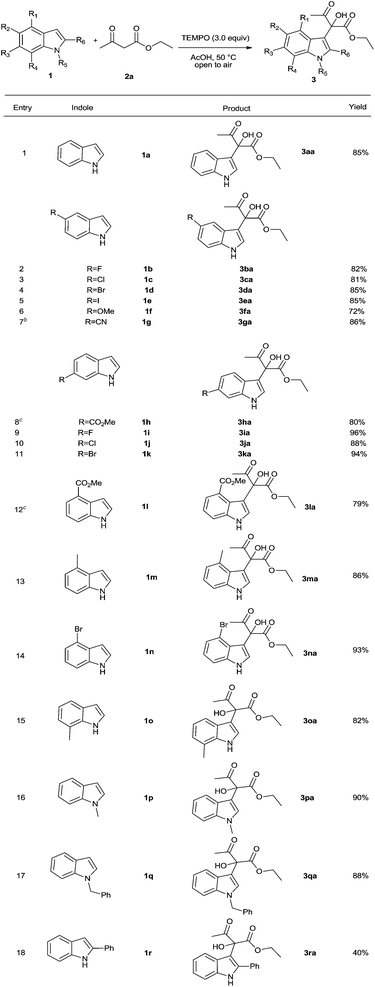
Next, the generality of this process was examined (Table 2). The results demonstrated that the domino multicomponent reaction is applicable to a wide range of indoles and, as a result, it enables ready synthetic access to diverse indole derivatives containing α-hydroxy-ketoester groups at their β-positions. Specifically, indoles possessing a variety of electronically different substituents, including halogen, alkyl, alkoxy, cyano and methoxy carbonyl groups at different positions on the arene ring undergo reaction to produce the corresponding substituted indoles in good to excellent yields (Table 2, entries 1–15). N-Methyl- and N-benzyl-indole also react with ethyl acetoacetate in the presence of TEMPO to form the coupling products in high yields (Table 2, entries 16–17). 2-Phenyl indole (1r), containing a bulky C-2 substituent, also reacts to form the corresponding alcohol 3ra in moderate yield (Table 2, entry 18). It should be pointed out is that this product (3ra) consists of two atropisomers due to the presence of the phenyl group limits free rotation about the C–C bond connecting the indole and hydroxyacetoacetate group (1H and 13C NMR spectra in ESI†).
β-Ketoesters, containing sterically different substituents, were also used to explore the scope of the new tandem reaction, conducted under optimized conditions. The results (Table 3) show that nature of the ester alkoxy group has only a moderate effect on the efficiency of this process (entries 1–3). Moreover, β-ketoester substrates containing ethyl and phenyl ketone moieties also react with indole to generate the corresponding alcohol products in moderate yields (Table 3, entries 4–5). Finally, tertiary alcohols are produced in moderate yields in TEMPO promoted reactions between indole and 1,3-diketones such as acetyl acetone and benzoylacetone (Table 3, entries 6–7).
| a Reaction conditions: indole 1a (117 mmol, 1.0 mmol), 1,3-dicarbonyl compounds 2 (1.1 mmol), and TEMPO (468 mg, 3.0 equiv.) in acetic acids (5.0 mL) open to air 50 °C, 1–3 h. |
|---|
 |
The feasibility of extending the scope of the new TEMPO promoted reactions to other heterocycles was explored. The results of this effort showed that pyrrole and N-methyl pyrrole also participate in the process to produce the corresponding α-substituted pyrroles 6aa and 6ba in 40% and 56% yield, respectively (Scheme 1).
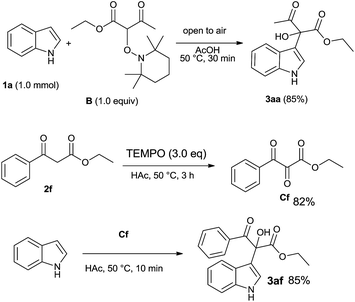
To gain preliminary insight into the mechanism of the new indole-tertiary alcohol forming reaction, we synthesized the TEMPO–ethyl acetoacetate adduct B14d (Scheme 2) and investigated its reaction with indole. We observed that stirring a solution of indole (1.0 mmol) and B (1.0 equiv.) at 50 °C for 30 min results in the formation of 3aa in 85% yield. Ethyl benzoylacetate (2f) reacted with TEMPO affording tricarbonyl compound Cf in 82% isolated yield. The 3af was obtained rapidly through the F–C reaction of indole and Cf by using acetic acid as solvent (Scheme 2).
This result suggests that a plausible mechanism for this process (Scheme 3) involves a pathway in which TEMPO disproportionation reaction, generating the N-oxopiperidinium ion A and N-hydroxypiperidine (TEMPOH), under acidic conditions, takes place initially. Accordingly, reaction between the active methylene compound and N-oxopiperidinium ion produces adduct B,19 which then undergoes loss of tetramethylpiperidine (TEMPH),14d,19 by a acidic elimination by the protonation route to form ethyl 2,3-dioxobutanoate C. Finally, in presence of acetic acid, through the Friedel–Crafts reaction of indole and vicinal tricarbonyl compounds C result in the adduct 3aa.17
In conclusion, a novel Friedel–Crafts reaction of indoles with vicinal tricarbonyl compounds generated in situ from 1,3-dicarbonyl compounds has been developed for direct preparation of indole and pyrrole substituted tertiary alcohols. The process, involving the disproportionation of TEMPO/α-aminoxylation of 1,3-dicarbonyl compounds/N–O bond cleavage to form the tricarbonyl intermediate/addition with indoles (C-3 position) or pyrroles (C-2 position), leads to the formation of indole-containing and pyrrole-containing tertiary alcohols in a domino one-pot manner. Key features of the methodology are that it tolerates a broad range of functional groups and it does not require the use of transition metals or an inert atmosphere. It is anticipated that the new process will serve as a general method for synthesis of tertiary alcohols that contain multiple functional groups. Further investigations are now underway to probe further the scope and applications of the method and to evaluate the biological properties of new substances produced in this effort.
Acknowledgements
This work was supported by the key project of Henan Provincial Department of Education (14A350006) and post-doctoral research projects of Henan Province (2013039).Notes and references
- J. C. Badenock and G. W. Gribble, Heterocyclic Scaffolds II: Reactions and Applications of Indoles, Springer Science & Business Media, 2010, vol. 2, p. 2 Search PubMed.
- For examples of selected reviews, see: (a) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed; (b) L. Joucla and L. Djakovitch, Adv. Synth. Catal., 2009, 351, 673 CrossRef CAS PubMed; (c) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS PubMed; (d) S. Cacchi and G. Fabrizi, Chem. Rev., 2011, 111, PR215 CrossRef PubMed.
- For selected reviews: (a) R. A. Sheldon, I. W. C. E. Arends, G.-J. ten Brink and A. Dijksman, Acc. Chem. Res., 2002, 35, 774 CrossRef CAS PubMed; (b) R. A. Sheldon and I. W. C. E. Arends, Adv. Synth. Catal., 2004, 346, 1051 CrossRef CAS PubMed; (c) T. Vogler and A. Studer, Synthesis, 2008, 1979 CAS; (d) L. Tebben and A. Studer, Angew. Chem., Int. Ed., 2011, 50, 5034 CrossRef CAS PubMed.
- (a) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2005, 127, 6968 CrossRef CAS PubMed; (b) M. Ghobrial, M. Schnürch and M. D. Mihovilovic, J. Org. Chem., 2011, 76, 8781 CrossRef CAS PubMed; (c) W. Su, J. Yu, Z. Li and Z. Jiang, J. Org. Chem., 2011, 76, 9144 CrossRef CAS PubMed; (d) E. Boess, C. Schmitz and M. Klussmann, J. Am. Chem. Soc., 2012, 134, 5317 CrossRef CAS PubMed; (e) D. B. Freeman, L. Furst, A. G. Condie and C. R. J. Stephenson, Org. Lett., 2012, 14, 94 CrossRef CAS PubMed; (f) J. Dhineshkumar, M. Lamani, K. Alagiri and K. R. Prabhu, Org. Lett., 2013, 15, 1092 CrossRef CAS PubMed; (g) H. Ueda, K. Yoshida and H. Tokuyama, Org. Lett., 2014, 16, 4194 CrossRef CAS PubMed; (h) J.-J. Zhong, Q.-Y. Meng, B. Liu, X.-B. Li, X.-W. Gao, T. Lei, C.-J. Wu, Z.-J. Li, C.-H. Tung and L.-Z. Wu, Org. Lett., 2014, 16, 1988 CrossRef CAS PubMed; (i) C.-J. Wu, J.-J. Zhong, Q.-Y. Meng, T. Lei, X.-W. Gao, C.-H. Tung and L.-Z. Wu, Org. Lett., 2015, 17, 884 CrossRef CAS PubMed.
- (a) F. Yang, J. Li, J. Xie and Z.-Z. Huang, Org. Lett., 2010, 12, 5214 CrossRef CAS PubMed; (b) J. Yang, Z. Wang, F. Pan, Y. Li and W. Bao, Org. Biomol. Chem., 2010, 8, 2975 RSC; (c) L. Huang, T. Niu, J. Wu and Y. Zhang, J. Org. Chem., 2011, 76, 1759 CrossRef CAS PubMed.
- (a) Y.-p. Zhu, M.-c. Liu, F.-c. Jia, J.-j. Yuan, Q.-h. Gao, M. Lian and A.-x. Wu, Org. Lett., 2012, 14, 3392 CrossRef CAS PubMed; (b) Q. Gao, J. Zhang, X. Wu, S. Liu and A. Wu, Org. Lett., 2015, 17, 134 CrossRef CAS PubMed.
- (a) J.-C. Wu, R.-J. Song, Z.-Q. Wang, X.-C. Huang, Y.-X. Xie and J.-H. Li, Angew. Chem., Int. Ed., 2012, 51, 3453 CrossRef CAS PubMed; (b) C. Huo, C. Wang, C. Sun, X. Jia, X. Wang, W. Chang and M. Wu, Adv. Synth. Catal., 2013, 355, 1911 CrossRef CAS PubMed; (c) R.-Y. Tang, X.-K. Guo, J.-N. Xiang and J.-H. Li, J. Org. Chem., 2013, 78, 11163 CrossRef CAS PubMed.
- (a) W. Wu and W. Su, J. Am. Chem. Soc., 2011, 133, 11924 CrossRef CAS PubMed; (b) J. Chen, B. Liu, D. Liu, S. Liu and J. Cheng, Adv. Synth. Catal., 2012, 354, 2438 CrossRef CAS PubMed; (c) L.-T. Li, J. Huang, H.-Y. Li, L.-J. Wen, P. Wang and B. Wang, Chem. Commun., 2012, 48, 5187 RSC; (d) L.-T. Li, H.-Y. Li, L.-J. Xing, L.-J. Wen, P. Wang and B. Wang, Org. Biomol. Chem., 2012, 10, 9519 RSC; (e) L. Zhang, C. Peng, D. Zhao, Y. Wang, H.-J. Fu, Q. Shen and J.-X. Li, Chem. Commun., 2012, 48, 5928 RSC; (f) X. Li, X. Gu, Y. Li and P. Li, ACS Catal., 2014, 4, 1897 CrossRef CAS; (g) B. Liu, J. Wang, B. Zhang, Y. Sun, L. Wang, J. Chen and J. Cheng, Chem. Commun., 2014, 50, 2315 RSC; (h) B. Zhang, B. Liu, J. Chen, J. Wang and M. Liu, Tetrahedron Lett., 2014, 55, 5618 CrossRef CAS PubMed.
- (a) M. V. Leskinen, K.-T. Yip, A. Valkonen and P. M. Pihko, J. Am. Chem. Soc., 2012, 134, 5750 CrossRef CAS PubMed; (b) M. V. Leskinen, Á. Madarász, K.-T. Yip, A. Vuorinen, I. Pápai, A. J. Neuvonen and P. M. Pihko, J. Am. Chem. Soc., 2014, 136, 6453 CrossRef CAS PubMed; (c) R. Y. Nimje, M. V. Leskinen and P. M. Pihko, Angew. Chem., Int. Ed., 2013, 52, 4818 CrossRef CAS PubMed.
- (a) Z. Zhang, Z. Hu, Z. Yu, P. Lei, H. Chi, Y. Wang and R. He, Tetrahedron Lett., 2007, 48, 2415 CrossRef CAS PubMed; (b) J. M. Lopchuk, W. L. Montgomery, J. P. Jasinski, S. Gorjifard and G. W. Gribble, Tetrahedron Lett., 2013, 54, 6142 CrossRef CAS PubMed.
- For selected reviews, see: (a) R. A. Sheldon, I. W. C. E. Arends, G.-J. ten Brink and A. Dijksman, Acc. Chem. Res., 2002, 35, 774 CrossRef CAS PubMed; (b) T. Vogler and A. Studer, Synthesis, 2008, 1979 CAS; (c) L. Tebben and A. Studer, Angew. Chem., Int. Ed., 2011, 50, 5034 CrossRef CAS PubMed.
- (a) T. Vogler and A. Studer, Org. Lett., 2007, 10, 129 CrossRef PubMed; (b) S. Kirchberg, T. Vogler and A. Studer, Synlett, 2008, 2841 CAS; (c) S. Kirchberg, R. Fröhlich and A. Studer, Angew. Chem., Int. Ed., 2009, 48, 4235 CrossRef CAS PubMed; (d) S. Kirchberg, R. Fröhlich and A. Studer, Angew. Chem., Int. Ed., 2010, 49, 6877 CrossRef CAS PubMed; (e) S. Kirchberg, S. Tani, K. Ueda, J. Yamaguchi, A. Studer and K. Itami, Angew. Chem., Int. Ed., 2011, 50, 2387 CrossRef CAS PubMed; (f) Z. He, S. Kirchberg, R. Fröhlich and A. Studer, Angew. Chem., Int. Ed., 2012, 51, 3699 CrossRef CAS PubMed; (g) Z. He, B. Wibbeling and A. Studer, Adv. Synth. Catal., 2013, 355, 3639 CrossRef CAS PubMed.
- For selected examples: (a) M. P. Sibi and M. Hasegawa, J. Am. Chem. Soc., 2007, 129, 4124 CrossRef CAS PubMed; (b) M. Pouliot, P. Renaud, K. Schenk, A. Studer and T. Vogler, Angew. Chem., Int. Ed., 2009, 48, 6037 CrossRef CAS PubMed; (c) K. Akagawa, T. Fujiwara, S. Sakamoto and K. Kudo, Org. Lett., 2010, 12, 1804 CrossRef CAS PubMed; (d) K. Akagawa, T. Fujiwara, S. Sakamoto and K. Kudo, Chem. Commun., 2010, 46, 8040 RSC; (e) T. Kano, H. Mii and K. Maruoka, Angew. Chem., Int. Ed., 2010, 49, 6638 CrossRef CAS PubMed; (f) J. F. van Humbeck, S. P. Simonovich, R. R. Knowles and D. W. C. MacMillan, J. Am. Chem. Soc., 2010, 132, 10012 CrossRef CAS PubMed; (g) S. P. Simonovich, J. F. van Humbeck and D. W. C. MacMillan, Chem. Sci., 2012, 3, 58 RSC; (h) Y. Li, M. Pouliot, T. Vogler, P. Renaud and A. Studer, Org. Lett., 2012, 14, 4474 CrossRef CAS PubMed.
- (a) U. Jahn, P. Hartmann, I. Dix and P. G. Jones, Eur. J. Org. Chem., 2002, 2002, 718 CrossRef; (b) M. Schämann and H. J. Schäfer, Synlett, 2004, 2004, 1601 Search PubMed; (c) E. Dinca, P. Hartmann, J. Smrček, I. Dix, P. G. Jones and U. Jahn, Eur. J. Org. Chem., 2012, 2012, 4461 CrossRef CAS PubMed; (d) P. Feng, S. Song, L.-H. Zhang and N. Jiao, Synlett, 2014, 25, 2717 CrossRef CAS.
- ESI†.
- (a) W. Zhuang, N. Gathergood, R. G. Hazell and K. A. Jørgensen, J. Org. Chem., 2001, 66, 1009 CrossRef CAS; (b) B. Török, M. Abid, G. London, J. Esquibel, M. Török, S. C. Mhadgut, P. Yan and G. K. S. Prakash, Angew. Chem., Int. Ed., 2005, 44, 3086 CrossRef PubMed; (c) Y. Hui, W. Chen, W. Wang, J. Jiang, Y. Cai, L. Lin, X. Liu and X. Feng, Adv. Synth. Catal., 2010, 352, 3174 CrossRef CAS PubMed; (d) G. Grach, A. Dinut, S. Marque, J. Marrot, R. Gil and D. Prim, Org. Biomol. Chem., 2011, 9, 497 RSC; (e) C. Wolf and P. Zhang, Adv. Synth. Catal., 2011, 353, 760 CrossRef CAS PubMed; (f) D. Carmona, M. P. Lamata, A. Sanchez, F. Viguri, R. Rodriguez, L. A. Oro, C. Liu, S. Diez-Gonzalez and F. Maseras, Dalton Trans., 2014, 43, 11260 RSC; (g) D. Carmona, P. Lamata, A. Sánchez, P. Pardo, R. Rodríguez, P. Ramírez, F. J. Lahoz, P. García-Orduña and L. A. Oro, Organometallics, 2014, 33, 4016 CrossRef CAS.
- (a) W. J. Moran, K. L. MacRory and A. Rodriguez, RSC Adv., 2012, 2, 8962 RSC; (b) B. C. Loosley, R. J. Andersen and G. R. Dake, Org. Lett., 2013, 15, 1152 CrossRef CAS PubMed; (c) J. Schütte, F. Kilgenstein, M. Fischer and U. Koert, Eur. J. Org. Chem., 2014, 5302 CrossRef PubMed.
- H. Li, Y.-Q. Wang and L. Deng, Org. Lett., 2006, 8, 4063 CrossRef CAS PubMed.
- M. Hayashi, M. Shibuya and Y. Iwabuchi, Synlett, 2012, 23, 1025 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization. CCDC 1056685. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra17383c |
| This journal is © The Royal Society of Chemistry 2015 |