The effect of zinc oxide (ZnO) addition on the physical and morphological properties of cellulose aerogel beads†
Abstract
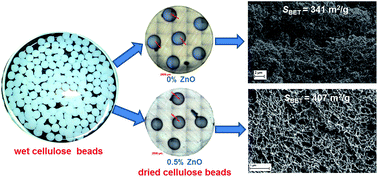
Microsized open porous beads of cellulose were made using the dissolution medium containing mixtures of 7 wt% NaOH and 12 wt% urea and additionally various concentrations of ZnO to study its effect on physical and morphological properties of the cellulose beads formed. It has been observed that such cellulose aerogel beads prepared with lower concentrations of ZnO show shrinkage while drying whereas beads prepared with higher concentrations of ZnO do not exhibit much shrinkage. The dried cellulose aerogel beads were spherical with diameters between 2 and 2.5 mm. The skeletal density of all dried cellulose beads was measured as 1.5 g cm−3. FT-IR spectra reveal that the structure of cellulose I transformed to cellulose II during dissolution and regeneration in a coagulation medium, which was also confirmed from XRD measurements. The beads prepared with a NaOH/urea/ZnO aqueous solution exhibit better thermal stability. We found that the addition of 0.5 wt% ZnO to the NaOH/urea mixture greatly increased the specific surface area of the cellulose beads up to 407 m2 g−1 compared to control cellulose beads (341 m2 g−1). SEM images indicate that a dense nano-fibrillar network structure was formed in the interior of the cellulose aerogel beads prepared with 0.5 wt% ZnO.


 Please wait while we load your content...
Please wait while we load your content...