Safflomin A inhibits neuraminidase activity and influenza virus replication
Abstract
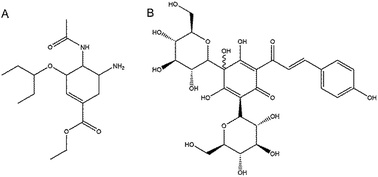
Neuraminidase (NA) is a glycoprotein on the surface of the influenza virus that plays an important role in the early processes of virus infection and viral release from the infected cells. NA inhibitors are currently the most effective drugs to treat influenza virus infection. Many traditional Chinese medicines (TCMs) in various formulations have been used in Chinese clinics to treat influenza, however, the effective constituents and the mechanism of action are mostly unknown. In this paper, we have tested almost 300 natural compounds from a Chinese medicinal herbal compound library to evaluate their anti-NA activities in vitro. Safflomin A (SA) was one of the compound detected with NA inhibitory activities. It showed inhibition against neuraminidases from H1N1 and H3N2 of type A and neuraminidase of type B influenza viruses. Enzyme kinetic tests using SA revealed that the types of inhibition against N1 and N2 neuraminidases were noncompetitive. The interaction of SA with the N1 and N2 neuraminidases was analyzed using molecular simulation and docking, which showed that SA was bound in the non-active sites of N1 and N2. SA was also analyzed for its cytotoxicity and anti-viral activities in cell culture. It showed inhibitory effect for viral replication of H1N1 and H3N2 influenza viruses in MDCK cells. Enzymatic analysis indicated that a SA and oseltamivir carboxylate combination treatment was synergistic with combination index (CI) values ranging between 0.49 and 0.52 against neuraminidase of A/New Caledonia/20/1999 (H1N1), and ranging between 0.56 and 0.88 against neuraminidase of A/Fujian/411/2002 (H3N2). These results suggest that an herbal formulation containing SA in combination with oseltamivir carboxylate may serve as a potential therapeutic option to currently available anti-influenza therapeutics.
- This article is part of the themed collection: Coronavirus articles - free to access collection

 Please wait while we load your content...
Please wait while we load your content...