Synthesis and characterization of UV-curable acrylate films modified by functional methacrylate terminated polysiloxane hybrid oligomers
Abstract
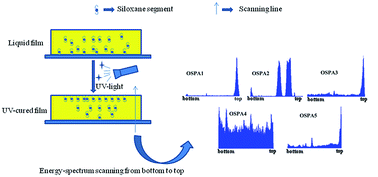
A series of novel methacrylate terminated polysiloxane hybrid oligomers and functional acrylate oligomers were synthesized and characterized by GPC, FT-IR and NMR. The functional polysiloxane oligomers were introduced into the acrylate UV-curing system to improve its surface and thermal properties. With increasing the organosiloxane content, the contact angles of the UV-cured films increased, suggesting that the organosiloxane segments migrated to the top surface. The SEM and EDS results demonstrated the migration of the organosiloxane segments. The refractive index results showed that the optical performance did not decrease after the organosiloxane segments were incorporated. According the TGA curves, the decomposition temperatures of the polysiloxane/acrylate composite UV-cured films were higher than that of the pure acrylate UV-cured film, which demonstrated that the organosiloxane groups enhanced the thermal properties of the acrylate film due to the high energy of the Si–C bond. The observation of the fractured-surface morphology showed that the organosiloxane segments floated on the surface of the UV-cured films.

 Please wait while we load your content...
Please wait while we load your content...