Nano-pesticide formulation based on fluorescent organic photoresponsive nanoparticles: for controlled release of 2,4-D and real time monitoring of morphological changes induced by 2,4-D in plant systems†
Abstract
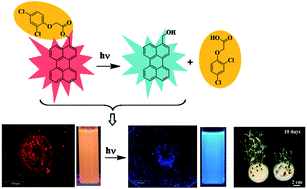
In recent times, nano-pesticide formulations have gained great popularity since they enable effective usage of smaller quantities of the pesticides without creating much damage to the environment. The benefits of a nano-pesticide formulation can be further expanded by adding to its arsenal both tracking ability and precise control over the pesticide release. Recently, fluorescent photoresponsive nanocarriers have gained considerable momentum in the field of drug delivery since they can act as both a “fluorophore” for cell luminescence imaging and a “phototrigger” for regulated drug release by external light stimuli. Hence, we report for the first time a nano-pesticide formulation based on fluorescent photoresponsive organic nanoparticles of perylene-3-ylmethanol for regulated release of pesticide 2,4-dichlorophenoxyacetic acid (2,4-D). Further, the fluorescent nature of the photoresponsive organic nanoparticles was used to study the morphological changes induced by 2,4-D inside the plant system using confocal imaging studies. Additionally, the fluorescent colour change by photoresponsive organic nanoparticles before and after photorelease was exploited for real time monitoring of 2,4-D release inside the plants. Bioassay experiments revealed that nano-pesticide, Pe-2,4-D efficiently delivered 2,4-D inside the plant tissues improving its herbicidal activity. Such photoresponsive multifunctional nanocarriers with good fluorescence, cellular uptake property and efficient photoregulated release ability will be of great benefit in the construction of nano-pesticide formulations.

 Please wait while we load your content...
Please wait while we load your content...