Influence of water on the retention of methyl tertiary-butyl ether by high silica ZSM-5 and Y zeolites: a multidisciplinary study on the adsorption from liquid and gas phase†
A. Martuccia,
I. Braschi*b,
C. Bisioc,
E. Sartid,
E. Rodegheroa,
R. Bagatine and
L. Pasti*d
aDepartment of Physics and Earth Sciences, University of Ferrara, Via G. Saragat 1, I-44123 Ferrara, FE, Italy
bDepartment of Agricultural Sciences, University of Bologna, Viale G. Fanin 44, I-40127 Bologna, BO, Italy
cDepartment of Sciences and Technological Innovation, University of Eastern Piedmont A. Avogadro, Viale T. Michel 11, I-15121 Alessandria, AL, Italy
dDepartment of Chemistry and Pharmaceutical Sciences, University of Ferrara, Via L. Borsari 46, I-44123 Ferrara, FE, Italy. E-mail: l.pasti@unife.it
eResearch Center for Non-Conventional Energy – Istituto Eni Donegani Environmental Technologies, via Maritano, 26, San Donato Milanese, MI I-20097, Italy
First published on 7th October 2015
Abstract
Ambient temperature adsorption isotherms have been determined for methyl tert-butyl ether (MTBE) in aqueous solutions on high silica ZSM-5 and Y zeolites which differ from each other in framework topology and pore window apertures. The comparison of their adsorption properties was performed to evaluate the importance of the competitive interference of water with pore size. The adsorption Henry's constants for MTBE decrease with an increase in the zeolite pore size, indicating that confinement of the adsorbate in the zeolite pores is a driving force for adsorption. On the contrary, the saturation capacity increases with an increase in the adsorbent pore size (i.e. about 95 and 250 mg g−1 for ZSM-5 and Y, respectively). These data were confirmed from Rietveld refinement, that clearly revealed the incorporation of about 8 and 32 MTBE molecules per ZSM-5 and Y unit cell respectively. X-ray diffraction and IR spectroscopy indicate that significant clustering of the guest molecules (i.e. water and MTBE) occurs in the two investigated zeolites during adsorption from both the liquid and gas phase. The clusters originate from hydrogen bonding between MTBE and water molecules and their interaction with zeolite lead to framework flexibility for both zeolites. However, the framework deformation in Y is more evident than in ZSM-5 and occurs with a change of the real symmetry. These findings could have an impact on the adsorption, separation and catalytic use of zeolites for oxygenate compounds.
1 Introduction
Methyl tert-butyl ether (MTBE, C5H12O) is an important oxygenated compound used as a gasoline additive to increase octane ratings and to improve combustion, thus reducing the emissions of carbon monoxide and hydrocarbons.1 This fuel additive is of particular concern in water pollution,2,3 due to its high aqueous solubility and high vapour pressure (about 50 g L−1 and 245 mmHg at 25 °C, respectively4,5). MTBE is toxic and is a potential human carcinogen,2 moreover it is not readily biodegradable in the natural environment. The U.S. Environmental Protection Agency (EPA) has placed MTBE on the drinking water contaminant candidate list for future and has estimated that its levels based on organoleptic effects should not exceed 20 μg L−1 in terms of odour and 40 μg L−1 in terms of taste.6 For these reasons different remediation technologies to reduce MTBE in the environment have been developed. In particular, those based on adsorption of MTBE on solid adsorbent seem to be particularly adequate, since it has been shown that oxidation based remediation can lead to the formation of by-products and background water-related oxidation by-products.7,8 Many studies have been conducted to highlight the ability of hydrophobic/organophilic zeolites to selectively extract organic compounds from water9–12 but only a few of them have been addressed the effect of water, either in liquid or in vapour phase, on their adsorption (e.g. see ref. 9 and 10). In this field, the works of Milestone and Bibby,9 Grose and Flanigen,10 and Anderson13 were pioneeristic for the introduction of hydrophobic zeolite as adsorbent materials for organic contaminants from water.14–16 In particular in ref. 12 and 13 it has been demonstrated that zeolite materials were more efficient that activate carbon for MTBE removal, and zeolite beta and mordenite exhibited good properties for MTBE adsorption.12,13,16,17 One of the main advantage of beta and mordenite zeolites over other adsorbent materials was that no competition among the organic matter, commonly dispersed in natural water, and MTBE molecules was observed.12,13 In addition, Arletti17 highlighted the presence of MTBE in zeolite microporosities by structure refinement performed using the Rietveld method. Among high silica (HS) zeolites, mordenite has been shown to be the best MTBE adsorbent also for gas phase systems.16Recent studies show that others HS zeolites also exhibit considerably large MTBE adsorption uptakes.18 The adsorption of MTBE has been also deeply investigated by Rossner and Knappe.14 In these studies, short bed adsorbent tests were carried out on both ultrapure and river water, to obtain parameters describing MTBE adsorption equilibria and kinetics. It has been found that, among the studied adsorbents, silicalite was the most cost-competitive adsorbent when considering both life time and usage rate of the adsorbent materials. Most of these studies were devoted to the optimal selection of an efficient adsorbent material based on the comparison of the MTBE uptake from aqueous solutions. However, criteria based solely on macroscopic observations are often not exhaustive for choosing the most efficient sorbent materials, and to gain a full understanding of the different behaviours of these materials. On the other hand, physicists specializing in surface science tend to favour adsorption directly from the gas phase.19 This is partly due to the need to simplify the system by avoiding the interference of solvent in modelling and theoretical interpretation.20 The results obtained in gas phase systems can be significantly different from those obtained for aqueous solutions, either to clearly evidence a particular adsorption mode, or to mimic what happens when the water activity is decreased by drying. However, it must be kept in mind that when the question has been studied, the presence of water did indeed interfere strongly with organic compounds adsorption modes.21–23 In fact, adsorption from aqueous solutions depends not only on the dimension of the pore systems, but also on the interactions among zeolite framework, the adsorbate and water molecules, as recently reported for the adsorption of dichloroethane (DCE) on mordenite,24 ZSM-5, and Y zeolites.25 Water molecules inside the porous framework lead to a multicomponent system instead of a single component adsorption mechanism and interact with DCE through hydrogen bonding. Interaction between sorbed molecules has also been reported by Krishna and van Baten21 in the adsorption of water–alcohol mixtures on zeolites and by Bai et al.20 for polyols–zeolite systems. The adsorption from organic–water mixtures markedly differs from that of their pure components and the differences have been ascribed to H-bonding between the adsorbates and zeolite framework. Since MTBE can interact with water through H-bonding as well, in the present study the adsorption of the binary MTBE–water system onto two different HS zeolite materials (i.e. ZSM-5 and Y) was studied in a broad range of conditions.
One of the main target of the present work is in fact to investigate whether the results obtained for chlorinated compounds and alcohols can be generalized to organic compound containing electronegative heteroatoms.
The two selected adsorbents were commercial ZSM-5 and Y zeolites: the possibility to find them on the market was considered a strong decision point for their selection. They differ from each other in structure and in the opening windows of the framework, and the comparison of their adsorption properties was performed to evaluate the importance of the competitive interference of water with pore size. ZSM-5 (MFI-type framework topology26) is a medium pore material whose framework is characterized by four symmetrically independent 10-membered rings (MR) of tetrahedra (in the P21/n or P212121 symmetries). Two of these delimit the sinusoidal channel (ZZ) (sinusoidal ring A and sinusoidal ring B, defined in Fig. 1A) and the other two confine the straight channel (SC) (SC ring A and SC ring B, Fig. 1A). The zeolite Y (FAU-type framework topology26) is a large pore material with large cavities (referred to as “supercages”), joined to four others via 12MR (see Fig. 1B).
 | ||
| Fig. 1 Unit cell parameters, Crystallographic Free Area (CFA, sensu Baerlocher26) and channel ellipticity (ε*) for ZSM-5 and Y before and after MTBE adsorption. ε* = ratio between the longest and the shortest pore dimensions. In the picture the T atom of the TO4 tetrahedron is located at each of the corners, and the oxygen atoms (not shown to aid clarity) are located towards the mid-points of the lines joining each T atom. | ||
The two selected materials are characterized by a similar silica/alumina (SiO2/Al2O3) ratio (SAR), however, since the adsorption could be influenced by both the polar (defect density) and nonpolar domains of the framework,27 the adsorption of MTBE from both batch aqueous solution and gas phase in the presence of water was studied and the interactions with the framework investigated.
2 Experimental
2.1 Materials and methods
The linearity of the method for the quantitative analysis was tested by evaluating the calibration curves: standard solutions of MTBE in MilliQ® (Millipore, MA, USA) water were analysed at varying concentration levels in the 0.1–15 and 10–300 mg L−1 ranges. Each concentration was analysed twice. The linearity range was evaluated, as well as the method detection limit (LOD) computed from the calibration line.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (mg mL−1) was employed. After equilibration, (24 h) at a temperature of 25.3 ± 0.5 °C under stirring, the solids were separated from the aqueous solution using centrifugation (10
4 (mg mL−1) was employed. After equilibration, (24 h) at a temperature of 25.3 ± 0.5 °C under stirring, the solids were separated from the aqueous solution using centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min) and analysed by HS-SPME-GC as described above.
000 rpm for 30 min) and analysed by HS-SPME-GC as described above.![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (for zeolite Y), and P21/n (for ZSM-5) space groups, respectively. Framework and extra-framework atomic coordinates, and temperature factors are provided as ESI.†
(for zeolite Y), and P21/n (for ZSM-5) space groups, respectively. Framework and extra-framework atomic coordinates, and temperature factors are provided as ESI.†3 Results and discussion
3.1 Adsorption isotherms from aqueous solutions
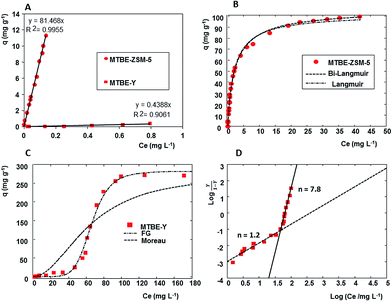
Fig. 2A shows MTBE adsorption isotherms on ZSM-5 and Y, obtained for dilute aqueous solutions at 25 °C. The aqueous phase MTBE concentrations in the μg L−1 range is interesting because it represents the level of concern for natural and drinking water. In Fig. 2A, it can be seen that both the isotherms follow a linear trend, and by comparing the slope of the linear equation it can also be noticed that Y is less efficient in MTBE removal from water solutions than ZSM-5. Additionally, a similar behaviour was observed for DCE adsorption on Y and ZSM-5.25 Similar trends have also been reported in the literature.30,31 On the contrary, the shape of the adsorption isotherms of MTBE on ZSM-5 and Y differ from each other when a wide range of concentrations is considered, (see Fig. 2B and C). In this case the aqueous MTBE concentrations are included the g L−1 range which is below the aqueous solubility (i.e. 50 g L−1) and might be encountered in the event of spill. In particular, in Fig. 2B and C, it can be seen that the adsorption data on ZSM-5 follow a type I, whereas, the Y data follow a type V isotherm according to IUPAC classification,32 and that Y zeolite adsorbed very effectively at higher concentrations. A similar behaviour has been already observed for the adsorption of DCE on these two zeolites.25 The type I isotherms are usually fitted by Langmuir, or heterogeneous extended Langmuir models:33
 | (1) |
 | (2) |
Table 1 lists the best adsorption isotherm parameters. To statistically compare the two model, the ratio of the Fisher parameter was calculated35 from the sum of the squares of the residuals (SSE, see Table 1). As suggested by Gritti,36 one isotherm model would be statistically better than the other one if the ratio of their two Fisher parameters would exceed 3.47, given their degrees of liberty d1 and d2 (d1 = 25 − 4 = 21, d2 = 25 − 2 = 23) and a level of risk α of 0.05. Since the Fisher parameter was 3.35, the bi-Langmuir and the Langmuir adsorption models seem to be statistically equivalent. In such a case, the adsorption data provide insufficient insight to the adsorption mechanisms and the choice between the two adsorption model should be based on the knowledge of the adsorption process.
| R2 | SSE | |||
|---|---|---|---|---|
| MTBE–ZSM-5 | Langmuir | |||
| qs | 94 | 0.9946 | 155.8 | |
| b | 0.498 | |||
| Bi-Langmuir | ||||
| qs,1 | 62 | 0.9959 | 118.1 | |
| b1 | 0.683 | |||
| qs,2 | 32 | |||
| b2 | 0.234 | |||
| MTBE–Y | Moreau | |||
| qs | 364 | 0.8926 | 4870 | |
| b | 0.067 | |||
| I | 61.12 | |||
| Hill | ||||
| qs | 281 | 0.9921 | 1800.3 | |
| KD | 73 | |||
| N | 6.5 | |||
| Fowler–Guggenheim | ||||
| KFG | 0.00153 | 0.9912 | 0.923 | |
| W | 5.805 | |||
Consequently, to gain information on the ZSM-5 adsorption sites a structural investigation was performed in order to localize the MTBE molecules in the ZSM-5 framework, the results are reported in Rietveld refinement section.
For that which concerns Y zeolite, on one hand, it was observed a higher saturation capacity than that of ZSM-5, probably related to the higher pore volumes of Y as well as to the different framework geometry, mesopores and/or framework defect density with respect to ZSM-5.27 Recently, Pasti et al.25 demonstrated that the adsorption properties of zeolitic materials are not only affected by the SAR and the size of the micropores but also by their structural features. On the other hand, the affinity at low MTBE concentrations to Y was lower than that to ZSM-5. The difference in affinity of the two zeolites indicates that different interaction types occur between MTBE and the zeolite framework. Reasonably, the difference in the adsorption properties between Y and ZSM-5 arises from the interference of water in the adsorption process. To investigate the adsorption process of MTBE, the experimental data were fitted by using various isotherm models. Several mathematical models can be used to describe adsorption data: among them, Moreau, Hill, Fowler–Guggenheim (F–G) isotherm models are those most frequently employed to fit S-shaped isotherms.37,38
The Moreau isotherm model describe the adsorption onto an homogeneous adsorbent surface on which adsorbate–adsorbate interactions take place,37 moreover, this model is nearly identical to the Ruthven model developed for adsorption on zeolites:39
 | (3) |
| I = exp(εAA/RT) | (4) |
The Hill isotherm model is employed to describe cooperative sorption mechanism:
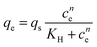 | (5) |
Finally, the F–G isotherm model, is the most employed equation in fitting adsorption data of organic compounds on zeolite and porous materials.42
 | (6) |
In Table 1 are reported the fitting parameters obtained for the three models tested. The experimental data was fitted with the linear form of the F–G equation, whereas non linear regression was carried out for the other two models. To evaluate the fractional coverage, in the F–G equation, the saturation capacity was evaluated by non linear fitting of the data with a shifted Langmuir model. The determination coefficient of the Moreau isotherm model is lower than the others, thus indicating a poor fitting of the model. In order to examine the models, the fitting equations and the experimental isotherm were plotted (see Fig. 2C). The variables in the F–G equation cannot be separated and, in this case, a simulated isotherm was calculated for each equilibrium concentration using a computer code. It can be noticed in Fig. 2C that both the F–G and the Hill model well describe the equilibrium isotherms. The interaction energy estimated from F–G model is positive thus indicating interaction of the adsorbate molecules.
Additionally the Hill plot of the data was calculated (see Fig. 2D) and the shape of the plot indicated a cooperative mechanism in the adsorption process (n = 7.8). Therefore both the models evidenced strong adsorbate–adsorbate interactions. To gain insight into the MTBE–Y system, a structural and spectroscopic investigation was carried out. Finally, by comparing the data of MTBE–Y system to those of the DCE–Y25 it seems that Y zeolite is selective for the least water-soluble compound. This finding has been observed also for the adsorption of VOCs in gas/solid adsorption.43
3.2 Rietveld refinement
Two crystallographically independent MTBE sites were localized: the first one (MTBE1 in Fig. 3A is located near the intersections of sinusoidal and straight 10MR channels (C1a, C2a, C3a, C4a, C5a, O1a sites in Table S1 ESI†) and the second one (MTBE2 in Fig. 3A) in the sinusoidal 10MR channel (C1b, C2b, C3b, C4b, C5b, O1b sites in Table S1 ESI†), respectively.
The occupancy factors indicate that the level of loading is 8 MTBE molecules per unit cell, Fig. 3A shows the high-loaded structure of ZSM-5-MTBE, with the guest molecules located in both channels, in common with other non-aromatic hydrocarbons.44–46 Although ZSM-5 is hydrophobic, difference Fourier maps revealed the presence of a further extra-framework site partially occupied by a low amount of water molecules (W site in Table S2 ESI†). On the whole, 8 MTBE molecules (corresponding to ∼11% zeolite dry weight (dw)) and about 2 water molecules (corresponding to about 0.5% zeolite dw) were detected. These species are related to the weight loss occurring in the 100–900 °C range of the thermal analysis (green line in Fig. 4A). As reported in the TG curves of Fig. 4A, the weight loss occurring below ∼100 °C can be ascribed to the elimination of species weakly bonded to the surface (∼5% zeolite dw). Above this temperature, the sudden slope change (see Fig. 4B) can be reasonably due to the release/decomposition of extra-framework species (MTBE and water molecules) trapped within the MFI pores (weight loss ∼11.5% dw). Total weight loss at 900 °C is 16.5% for ZSM-5-MTBE, in very good agreement with the refined occupancies as well as the adsorption data. In the same temperature range, the weight loss of the as-synthesized material is equal to 5.4% and can be ascribed to the condensation of nest silanol groups.
The refined distances between the framework oxygen, water oxygen atoms and MTBE molecules suggested the occurrence of MTBE–water complexes interacting with the framework (Fig. 3A). In particular, W site can interact with three O framework oxygens as well as with MTBE molecules in both MTBE1 and MTBE2 sites (W–O25 = 2.61 Å, W–O31 = 2.97 Å, W–O44 = 2.58 Å, W–C2b = 3.08 Å, W–C2b = 2.95 Å, W–C3b = 2.95 Å), thus acting as a bridge between MTBE and the framework. The refined distances of the MTBE oxygen atom from the framework oxygen atoms (O5a–O20 = 2.63 Å, O5a–O24 = 3.28 Å, O5a–O29 = 3.34 Å) suggest that MTBE molecules could interact with the framework. The importance of these interactions in stabilizing the guest structure within the zeolite host framework might therefore need reconsideration. The presence of organic compound–water molecule complexes (clusters or short chains) strongly interacting with the framework has also been recently observed in HS mordenite,24,25 ferrierite18 as well as in the same Y and ZSM-5 zeolite after DCE adsorption from aqueous solutions.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) (Fig. 1B). The tert-butyl group is given by C1 and O2/C2 respectively, and the methoxylic group formed O2/C2 and C3 sites, respectively.
(Fig. 1B). The tert-butyl group is given by C1 and O2/C2 respectively, and the methoxylic group formed O2/C2 and C3 sites, respectively.A further extra-framework site obtained from Difference Fourier Maps was attributed to water molecules (W site in Fig. 3B). As already observed for MTBE, water molecules can assume six different orientations, one for each MTBE positions (Fig. 3B), thus indicating a cooperative mechanism in the adsorption. The refined distances of the oxygen atom in the water molecules from themselves (2.96 Å) and from the O1 framework oxygen (3.16 Å) suggest that water molecules are connected to each other to form W–W water chains (C2–W = 3.10 Å, C3–W = 2.38 Å, 2.54 Å, and 3.05 Å). After MTBE and water adsorption, changes in the unit cell volume, as well as crystallographic free area and ellipticity of the 12MR channel (Fig. 1B) with respect to the as-synthesized material are highlighted. On the whole, the extra-framework content corresponds to about 11 water (∼6% zeolite dw) and 32 MTBE molecules (corresponding to ∼31% zeolite dw) per unit cell. These species are related to the weight loss occurring in the 100–900 °C range of the thermal analysis (∼39% zeolite dw, Fig. 4A), in good agreement with the adsorption data.
3.3 Infrared spectroscopy: MTBE–H2O adsorption from gas phase
According to the results on adsorption trials conducted in water, the MTBE retention by both HS zeolites is achieved through the involvement of water molecules that eventually stabilize MTBE in adsorbed form through the formation of MTBE–H2O clusters at interaction distances with the zeolite framework. In this section, the effect of water on MTBE adsorption via gas phase was investigated through IR analysis of MTBE/H2O equimolar mixture. The adsorption of each single component was also reported for comparison, as well as the features of the two bare zeolites.When ZSM-5 treated at RT was contacted with 8 mbar of water (Fig. 5A), the bands of isolated silanols disappeared and a complex signal dominated by three new main maxima at ca. 3650, 3420, and 3240 cm−1 became visible in the region of H-bonded OH stretching, thus indicating the presence of water molecules differently H-bonded to the zeolite silanols. Since the three signals were not significantly revealed when water was contacted with ZSM-5 treated at 500 °C (Fig. 5B), it was deduced that nest silanols, and only to a minor extent, isolated silanols are involved in the water adsorption process by the zeolite. Unfortunately, the position of the bending mode of water OH groups was not clearly detectable because overlapped to a band at ca. 1650 cm−1, an overtone of the zeolite framework vibration. In addition, the region of OH stretchings shows new components in the 3740–3000 cm−1 region with respect to the bare zeolite, thus indicating H-bonding/polar interactions between MTBE and ZSM-5 silanols.
Adsorbed MTBE shows a complex signal formed by four main bands at 2977, 2943, 2915, and 2829 cm−1, which have been already assigned to stretching modes of the two different methyl groups of methoxyl and tert-butyl moieties.16,27 In the cited studies, a detailed description of the type and strength of the host–guest interactions between MTBE and polar (silanols) or nonpolar (siloxane framework) domains in HS zeolites can be found, as well as a description of the guest–guest interactions. In our study, when ZSM-5 was contacted with a equimolar mixture of MTBE and water (16 mbar, Fig. 5A), the spectral features of both components could be found, thus indicating their simultaneous adsorption on ZSM-5, which is seemingly able to retain a similar amount of MTBE both in the absence and in the presence of water. Here, a few considerations can be withdrawn: the intensity and the positions of both MTBE and water bands are similar to what it was observed when they were singly adsorbed. In addition, when the loaded MTBE–water mixture was desorbed at 2 mbar, the water signals were significantly reduced whereas those of MTBE were almost completely preserved, thus indicating that water can be released more easily than MTBE molecules.
Due to the complexity of the spectral signals in the OH stretching region (3750–3000 cm−1), which are informative of possible host–guest interactions with the zeolite outgassed at RT, no clear information on possible MTBE–H2O clusters inside the pores was obtained. A better observation of the region was achieved on ZSM-5 previously treated at 500 °C.
Fig. 5B shows the adsorption of 10 mbar of the equimolar mixture. Interestingly, here the complex signal in the OH stretching region shows maxima at ca. 3650, 3420, and 3240 cm−1 which are not visible in the zeolite singly loaded with water or MTBE, thus revealing the occurrence of H-bonded MTBE–water clusters. Also in this case, the adsorption of water is highly reversible, conversely to MTBE that remains entrapped when the pressure was reduced to 2 mbar.
These findings are in agreement to what it was obtained by the Rietveld analysis of XRPD data of the zeolite loaded with MTBE from water solution where water molecules are found stabilized by interactions with MTBE and the zeolite framework. Likely, the extra-stabilization of van der Waals forces acting on the organic component of H-bonded MTBE–water cluster, forces water to stay at interaction distance to the zeolite framework.
After interaction with pure water (8 mbar), a partial reduction of the band at 3737 cm−1 was observed, thus indicating a uncomplete reactivity/accessibility of isolated silanols to water molecules. In addition, a new component centered at ca. 3430 cm−1, assigned to stretching modes of H-bonded OH groups belonging to both the zeolite and water, could be observed. At lower frequencies, the bending mode of water OH groups at ca. 1630 cm−1 could be also found. When MTBE (8 mbar) was singly adsorbed on the zeolite Y, a new complex band at ca. 3290 cm−1 became visible beside the occurrence of MTBE bands between 3000 and 2800 cm−1, thus indicating the interaction of silanol groups with MTBE molecules. A detailed description of the polar and nonpolar host–guest interactions between MTBE and the zeolite Y has been already given elsewhere.27 When the zeolite was exposed to a equimolar MTBE–H2O mixture (16 mbar), beside the occurrence of MTBE bands, the OH stretching and bending regions resembled the spectral features of both components, thus revealing their co-adsorption. Interestingly, the water OH bending at 1638 cm−1 is found slightly upshifted (+7 cm−1) with respect to the same signal of singly adsorbed water at 1631 cm−1. This indicates that co-adsorbed MTBE molecules affect the chemical surrounding of water molecules. According to the H-bonding theory,48 the blue shift of the OH bending is associated to a ten/sevenfold redshift of the stretching signal of the related OH groups.
Unfortunately, the complexity of this region in our spectrum did not allow to clearly define the stretching component associated to the OH of water molecules H-bonded to MTBE. However, when the zeolite loaded with the mixture was outgassed to 2 mbar, both the signal at 1638 cm−1 and the component at 3431 cm−1 simultaneously disappeared, being thus referred to the same H-bonded water species.
Similar features were found in the IR spectrum of the mixture (13 mbar) contacted with the zeolite previously outgassed at 700 °C (Fig. 6B). Here, the nested silanols possibly interacting with MTBE or H2O were removed by condensation, and the band at 1635 cm−1, assigned to the bending mode of water OH groups, was shifted of +15 cm−1 with respect to the same signal (1620 cm−1) of singly adsorbed water (6 mbar). Finally, as far as the stability of the mixture components retained inside the Y porosities, when the zeolite loaded with the equimolar MTBE/water mixture (Fig. 6A) was outgassed to 2 mbar, water was easily desorbed by the HS zeolite whereas MTBE was partially retained.
4 Conclusions
The adsorption of MTBE onto HS MFI and FAU zeolites was investigated under different conditions: by batch adsorption and X-ray powder diffraction analyses from aqueous solution, and by infrared spectroscopy from gas phase in the presence of water. MTBE exhibits a type-I isotherm on MFI and a type-V isotherm on FAU, thus indicating a different interaction mechanism. In particular, the adsorption of MTBE on Y seems to be cooperative, thus involving adsorbate–adsorbate interactions. The adsorption Henry's constants for MTBE decreases with an increase in the zeolite pore size, indicating that confinement of the adsorbate in the zeolite pores is a driving force for adsorption. On the contrary, the saturation capacity increases with an increase in the adsorbent pore size (i.e. about 95 and 250 mg g−1 for ZSM-5 and Y, respectively). These data were confirmed from Rietveld refinement, that clearly revealed the incorporation of about 8 and 32 MTBE molecules per ZSM-5 and Y unit cell respectively. X-ray diffraction and IR spectroscopy indicate that significant clustering of the guest molecules (i.e. water and MTBE) occurs in the two investigated zeolites during adsorption from both liquid and gas phase. The clusters originate from H-bonding between MTBE and water molecules and their interaction with zeolite leads to framework flexibility for both zeolites. Water plays an important role in the adsorption process of MTBE from gas phase as well, with no significant reduction of retention capacity for both zeolites. These findings can be of a certain interest for scientists working with PRB technologies as well as with the decontamination of wet indoor environments from oxygenated organic compounds.Acknowledgements
The authors thank the Research Centre for Unconventional Energies, Istituto ENI G. Donegani – Environmental Technologies (San Donato Milanese (MI) Italy) for financial support.Notes and references
- J. Fenger, Atmos. Environ., 2009, 43, 13 CrossRef CAS PubMed.
- OEHHA (Office of Environmental Health Hazard Assessment), 1999 in Drinking Water, Pesticide and Environmental Toxicology Section, California EPA, USA, http://oehha.ca.gov/water/phg/pdf/mtbe_f.pdf.
- A. Amberg, E. Rosner and W. Dekant, Toxicol. Sci., 2001, 61, 62 CrossRef CAS.
- D. Mackay, W. Y. Shiu, K. C. Ma and S. C. Lee, Illustrated handbook of physical–chemical properties and environmental fate for organic chemicals—volatile organic chemicals, Taylor & Francis Group, LLC., 2006, vol. III, pp. 1–925 Search PubMed.
- P. Squillace, J. Pankow, N. Kortes and J. Zogorski, Environ. Toxicol. Chem., 2009, 16(9), 1836 CrossRef.
- US EPA in Washington, DC, US Environmental Protection Agency, 1997, pp. 11–13 (EPA-822-F-97-009), http://www.epa.gov/ost/drinking/mtbe.pdf.
- P. Chang and T. Young, Water Res., 2000, 34, 2233 CrossRef CAS.
- J. L. Graham, R. Striebich, C. L. Patterson, E. Radha Krishnan and R. C. Haught, Chemosphere, 2004, 54, 1011 CrossRef CAS PubMed.
- N. B. Milestone and D. M. Bibby, J. Chem. Technol. Biotechnol., 1981, 31(1), 732 CrossRef CAS PubMed.
- R. W. Grose and E. M. Flanigen, Patent US 4257885 A, 1981.
- A. O. Yazaydin and R. W. Thompson, J. Phys. Chem. B, 2006, 110, 14458 CrossRef CAS PubMed.
- L. Abu-Laila, J. A. Bergendahl and R. W. Thompson, J. Hazard. Mater., 2010, 1–3, 363 CrossRef PubMed.
- M. A. Anderson, Environ. Sci. Technol., 2000, 34, 725 CrossRef CAS.
- A. Rossner and D. R. Knappe, Water Res., 2008, 42, 2287 CrossRef CAS PubMed.
- L. Shiguang, V. A. Tuan, R. D. Noble and J. L. Falconer, Environ. Sci. Technol., 2003, 37, 4007 CrossRef.
- V. Sacchetto, G. Gatti, G. Paul, I. Braschi, G. Berlier, M. Cossi, L. Marchese, R. Bagatin and C. Bisio, Phys. Chem. Chem. Phys., 2013, 15, 13275 RSC.
- R. Arletti, A. Martucci, A. Alberti, L. Pasti, M. Nassi and R. Bagatin, J. Solid State Chem., 2012, 194, 135 CrossRef CAS PubMed.
- A. Martucci, L. Leardini, M. Nassi, E. Sarti, R. Bagatin and L. Pasti, Mineral. Mag., 2014, 78, 1161 CrossRef PubMed.
- M. Thommes, Studies in Surface Science and Catalysis, 3rd revised version, ed. J. Cejka, H. van Bekkum, A. Corma and F. Schüth, 2007, vol. 168, p. 495 Search PubMed.
- P. Bai, M. Tsapatsis and J. I. Siepmann, Langmuir, 2012, 28(44), 15566 CrossRef CAS PubMed.
- R. Krishna and J. M. van Baten, Langmuir, 2010, 26, 10854 CrossRef CAS PubMed.
- E. E. Mallon, M. Y. Jeon, M. Navarro, A. Bhan and M. Tsapatsis, Langmuir, 2013, 29(22), 6546 CrossRef CAS PubMed.
- J. I. Siepmann and A. E. Clark, J. Phys. Chem. C, 2014, 118(34), 19723 Search PubMed.
- A. Martucci, L. Pasti, M. Nassi, A. Alberti, R. Arletti, R. Bagatin, R. Vignola and R. Sticca, Microporous Mesoporous Mater., 2012, 151, 358–367 CrossRef CAS PubMed.
- L. Pasti, A. Martucci, M. Nassi, A. Cavazzini, A. Alberti and R. Bagatin, Microporous Mesoporous Mater., 2012, 160, 182 CrossRef CAS PubMed.
- C. Baerlocher, W. M. Meier and D. H. Olson, Atlas of Zeolite Framework Types, Elsevier, Amsterdam, 5th edn, 2001 Search PubMed.
- I. Braschi, G. Gatti, C. Bisio, G. Berlier, V. Sacchetto, M. Cossi and L. Marchese, J. Phys. Chem. C, 2012, 116, 6943 CAS.
- A. C. Larson and R. B. Von Dreele, General Structure Analysis System (GSAS), Los Alamos National Laboratory Report LAUR, 2000, p. 86 Search PubMed.
- B. H. Toby, J. Appl. Crystallogr., 2001, 34, 210 CrossRef CAS.
- J. Farrell, C. Manspeaker and J. Luo, Microporous Mesoporous Mater., 2003, 59, 205 CrossRef CAS.
- S. Kleineidam, C. Schüth and P. Grathwohl, Environ. Sci. Technol., 2002, 36, 4689 CrossRef CAS.
- K. S. W. Sing, Pure Appl. Chem., 1985, 57(4), 603–619 CrossRef CAS.
- B. A. de Moor, M.-F. Reyniers, O. C. Gobin, J. A. Lercher and G. B. Marin, J. Phys. Chem. C, 2011, 115, 1204 CAS.
- D. G. Ruthven, Principles of adsorption and adsorption processes, Wiley-Interscience; John Wiley & Sons, N.Y. (USA), 1984 Search PubMed.
- D. L. Massart, B. G. M. Vandengiste, S. N. Deming, Y. Michotte and L. Kaufman, Chemometrics: A Textbook, Elsevier, Amsterdam, 2nd edn, 1988 Search PubMed.
- F. Gritti, W. Piatkowskia and G. Guiochon, J. Chromatogr. A, 2002, 978(1–2), 81 CrossRef CAS.
- F. Gritti and G. Guiochon, Anal. Chem., 2004, 76, 4779 CrossRef CAS PubMed.
- K. Y. Foo and B. H. Hameed, Chem. Eng. J., 2010, 156, 2 CrossRef CAS PubMed.
- D. G. Ruthven, Principles of adsorption and adsorption processes, Wiley-Interscience; John Wiley & Sons, N.Y. (USA), 1984 Search PubMed.
- S. Goutellea, M. Maurinc, F. Rougierb, X. Barbautb, L. Bourguignon, M. Ducherb and P. Maire, Fundam. Clin. Pharmacol., 2008, 22, 633 CrossRef PubMed.
- H. Sato, W. Kosaka, R. Matsuda, A. Hori, Y. Hijikata, R. V. Belosludov, S. Sakaki, M. Takata and S. Kitagawa, Science, 2014, 343, 167 CrossRef CAS PubMed.
- O. Hamdaouia and E. Naffrechoux, J. Hazard. Mater., 2007, 147, 381 CrossRef PubMed.
- Y. J. Ho, D. K. Choi and S. H. Kim, Adsorption of organic solvent vapors on hydrophobic Y-type zeolite, AIChE J., 1998, 44, 1344 CrossRef PubMed.
- S. Fujiyama, N. Kamiya, K. Nishi and Y. Yokomori, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2014, 70, 856 CAS.
- S. Fujiyama, S. Seino, N. Kamiya, K. Nishi, K. Yoza and Y. Yokomori, Phys. Chem. Chem. Phys., 2014, 16, 15839 RSC.
- J. O. Titiloye, S. C. Parker, F. S. Stone and C. R. A. Catlow, J. Phys. Chem., 1991, 95, 4038 CrossRef CAS.
- L. Smith, A. K. Cheetham, R. E. Morris, L. Marchese, J. M. Thomas, P. A. Wright and J. Chen, Science, 1996, 271, 799 CrossRef CAS.
- S. Coluccia, L. Marchese and G. Martra, Microporous Mesoporous Mater., 1999, 30, 43 CrossRef CAS.
- A. Novak, Struct. Bonding, 1974, 18, 177 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Pattern diffraction patterns of bare and MTBE loaded zeolites (ZSM-5 and Y). Framework and extra-framework atomic coordinates, and temperature factors are provided (Table S1 and 2 ESI). See DOI: 10.1039/c5ra15201a |
| This journal is © The Royal Society of Chemistry 2015 |