ortho-Alkenylation of anilines with aromatic terminal alkynes over nanosized zeolite beta†
Naresh Mamedaab,
Swamy Perakaab,
Srujana Kodumurib,
Durgaiah Chevellab,
Mahender Reddy Marrib and
Narender Nama*ab
aAcademy of Scientific and Innovative Research, CSIR-Indian Institute of Chemical Technology, Hyderabad, Telangana, India
bI&PC Division, CSIR-Indian Institute of Chemical Technology, Hyderabad-500007, Telangana, India. E-mail: narendern33@yahoo.co.in; nama@iict.res.in
First published on 10th September 2015
Abstract
A simple, efficient and environmentally benign catalytic system has been successfully developed for highly regioselective electrophilic alkenylation of anilines with aromatic terminal alkynes over nanosized zeolite beta, which exhibits the highest catalytic activity among other conventional zeolites. The scope of the reaction was explored with various anilines and alkynes. However, the substrates (aniline or alkynes) with strong electron-withdrawing groups failed to react under the present catalytic conditions (except 4-nitroaniline). Moreover, the catalyst is recyclable and can be reused without significant loss in its catalytic activity.
Catalytic electrophilic aromatic substitution is one of the most useful approaches for the incorporation of carbon substituents onto aromatic rings.1 In particular, the ortho-alkenylation of aromatic amines with carbon–carbon multiple bonds is of significant importance. However, it is not an easily accessible process under normal Friedel–Crafts conditions2 due to the coordination of the Lewis acid with the nitrogen atom of the amino group, which leads to the deactivation of aromatic ring. Anilines substituted at the ortho position with an alkenyl groups (2-(1-phenylethenyl)anilines) are important precursors to a variety of heterocycles including indoles, quinolines and cinnolines, which are key structural units for a variety of biologically important compounds.3 They have also been used as synthetic intermediates in several total synthetic methods.4 Usually anilines with phenylacetylene gave the enamines in the presence of Lewis acids.5 To the best of our knowledge, there is only one report available in the literature on ortho-alkenylation of aromatic amines with phenylacetylene. However, main drawbacks associated with this method are lack of substrate scope (only limited to phenylacetylene) and the recyclability of catalyst was not studied.6 Thus, there is a much room to develop a simple, efficient and eco-friendly catalytic system for ortho-alkenylation of anilines with aromatic terminal alkynes.
In recent years, there is a substantial interest to design heterogeneous catalytic systems towards eco-friendly, clean and shape selective reactions. Catalysts based on various zeolites have main importance both in the petroleum and fine chemical industries,7 due to their unique physical and chemical properties, such as uniform channel size, large internal surface area, unique molecular shape selectivity, strong acidity and good thermal/hydrothermal stability. The large pore zeolite like Hβ has received much attention because of the large available micropore volume, large-pore channel system and the existence of active sites in diverse concentrations that are needful in a number of acid-catalyzed reactions.8
However, zeolites often show inadequate activity and/or fast deactivation because of poor diffusion efficiency.9 The slow transport in the zeolite micropores leads to unwanted secondary side reactions or slow reaction rates. In order to get benefit fully from the unique sorption and shape-selectivity effects in the micropores, the diffusion path length in the micropores should be extremely small.10 Nanosized zeolite crystals with narrow particle size distributions and sizes less than 100 nm have received much interest due to their great potential applications in catalysis and adsorption. The reduction of particle size from the micrometer to the nanometer scale leads to significant changes in the material properties, such as high external surface areas, reduced diffusion path lengths and more exposed active sites.11 In continuation of our efforts toward the development of novel and eco-friendly synthetic protocols using zeolites,12 we wish to describe here a simple and efficient catalytic method for ortho-alkenylation of anilines with aromatic terminal alkynes over nanosized zeolite beta. Nanosized zeolite beta was prepared according to the procedure described in our earlier report, which was systematically characterized by various spectroscopic techniques, such as, X-ray diffraction (XRD), Scanning Electronic Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FT-IR), NH3-TPD and 27Al NMR spectroscopy.13
Initially, we investigated the suitable reaction conditions for ortho-alkenylation of anilines with aromatic terminal alkynes using aniline and phenylacetylene as a model system (Table 1). In order to choose the best catalyst, the reaction was carried out over various zeolites and MCM-41 at 120 °C in sealed vial for 3 h in toluene (Table 1). Among the catalysts examined, Hβ zeolite showed the higher catalytic activity and furnished the 2-(1-phenylethenyl)aniline (3a) in 61% yield (Table 1, entry 5). Under the same reaction conditions, H-mordenite and HY provided the 3a in ≤30% yield (Table 1, entries 2 and 3). HZSM-5, MCM-41 and NaY delivered ≤15% yield of 3a (Table 1, entries 1, 4 and 6). Then, we tested the reaction with nanosized zeolite beta under similar reaction conditions, which afforded the corresponding product in highest yield (90%) compared to other catalysts (Table 1, entry 7). The best performance of nanosized zeolite beta is probably attributable to the higher acidity of nanosized zeolite beta (412 μmol g−1) than the Hβ zeolite ca. 269 μmol g−1.13
| Entry | Catalyst | Conversionb 1a (%) | Selectivityb 3a (%) | 3a Yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (2 mmol), 2a (2 mmol), catalyst (100 mg), toluene (1 mL), 120 °C, 3 h, sealed vial.b Conversion and selectivity based on GC.c Isolated yields.d Catalyst (50 mg).e Catalyst (25 mg).f 110 °C.g 100 °C.h 130 °C. | ||||
| 1 | HZSM-5 | 17 | 93 | 15 |
| 2 | H-mordenite | 23 | 98 | 22 |
| 3 | HY | 35 | 93 | 30 |
| 4 | NaY | 5 | 96 | 3 |
| 5 | Hβ | 65 | 98 | 61 |
| 6 | MCM-41 | 14 | 91 | 11 |
| 7 | Nanosized zeolite beta | 96 | 97 | 90 |
| 8 | Absence of catalyst | 00 | — | — |
| 9 | Nanosized zeolite beta | 56 | 98 | 52d |
| 10 | Nanosized zeolite beta | 23 | 98 | 20e |
| 11 | Nanosized zeolite beta | 66 | 99 | 62f |
| 12 | Nanosized zeolite beta | 50 | 97 | 46g |
| 13 | Nanosized zeolite beta | 95 | 98 | 91h |
Once nano beta was found as the best catalyst for the ortho-alkenylation of aniline with phenylacetylene, the influence of temperature was studied. The reaction was remarkably accelerated by varying the reaction temperature from 100 to 120 °C and the yield was enhanced from 46 to 90% (Table 1, entries 7 and 11–13) probably due to the particles move faster and collide more frequently on the catalyst surface as increase in the reaction temperature. The present reaction was also conducted with different amounts of catalyst and it was found that 100 mg of catalyst leads to the best result (Table 1, entry 7). The desired product was not observed in the absence of catalyst, thus confirming the role of the catalyst in the reaction (Table 1, entry 8). After extensive screening, we have observed that 100 mg of nanosized zeolite beta was optimum for 2 mmol of aniline and 2 mmol of phenylacetylene in order to get the maximum yield of the desired product in toluene (1 mL) at 120 °C (Table 1, entry 7).
Having the optimized conditions in hand, we investigated the versatility of this methodology by reacting the various anilines with phenylacetylene and the results are summarized in Table 2. Various electron-donating and electron-withdrawing groups were well tolerated with the present catalytic system and gave the desired products 3a–3j, 3m and 3o in low to excellent yields (Table 2, entries 1–15). In all cases, the complete ortho-regioselectivity was observed. Aniline produced the corresponding ortho-alkenylated product with 90% yield in 3 h (Table 2, entry 1). Aniline substituted with activating groups reacted smoothly and furnished the respective ortho-alkenylated products in good to excellent yields under these reaction conditions (Table 2, entries 2–6). However, the ortho-substituted anilines show relatively lower yields with respect to the corresponding para isomer probably due to some steric hindrance (Table 2, entries 4, 5 and 8). Halo substituted anilines, such as 4-chloroaniline (1g), 2-bromoaniline (1h) and 4-bromoaniline (1i) could be transformed into the corresponding 2-(1-phenylethenyl)anilines 3g–3i in 70–89% yield (Table 2, entries 7–9). In case of highly deactivating anilines, such as 4-nitroaniline (1j), 4-aminobenzamide (1k) and 4-aminobenzoicacid (1l), the corresponding products were obtained in low or zero yields (Table 2, entries, 10–12). The N-alkylated aniline 1m afforded the respective product 3m with 65% yield (Table 2, entry 13), whereas N-disubstituted aniline i.e., N,N-dimethylaniline (3n) failed to react under similar reaction conditions (Table 2, entry 14). Polycyclic aromatic amine 1o was also reacted smoothly to provide the corresponding ortho-alkenylated product 3o in 86% yield (Table 2, entry 15).
| Entry | R | R1 | R2 | Conversionb 1a–1o (%) | Selectivityb 3a–3o (%) | Product; yieldc (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1a–1o (2 mmol), 2a (2 mmol), nanosized zeolite beta (100 mg), toluene (1 mL), 120 °C, 3 h, sealed vial.b Conversion and selectivity based on GC.c Isolated yields. | ||||||
| 1 | H | H | H | 95 | 97 | 3a; 90 |
| 2 | 4-Meo | H | H | 98 | 99 | 3b; 96 |
| 3 | 4-Me | H | H | 97 | 98 | 3c; 93 |
| 4 | 2-Meo | H | H | 90 | 98 | 3d; 85 |
| 5 | 2-Me | H | H | 79 | 98 | 3e; 75 |
| 6 | 2,4-Me,Me | H | H | 84 | 98 | 3f; 78 |
| 7 | 4-Cl | H | H | 94 | 98 | 3g; 89 |
| 8 | 2-Br | H | H | 76 | 98 | 3h; 70 |
| 9 | 4-Br | H | H | 93 | 97 | 3i; 86 |
| 10 | 4-NO2 | H | H | 36 | 97 | 3j; 32 |
| 11 | 4-CONH2 | H | H | 00 | — | 3k; — |
| 12 | 4-COOH | H | H | 00 | — | 3l; — |
| 13 | H | Me | H | 69 | 98 | 3m; 65 |
| 14 | H | Me | Me | 00 | — | 3n; — |
| 15 | 3,4-Anthryl | H | H | 91 | 97 | 3o; 86 |
To extend the scope of this reaction further, we investigated the reaction of other aromatic terminal alkynes with aniline and the results are summarized in Table 3. Activating group present on aromatic ring of phenylacetylene i.e., 1-ethynyl-4-methylbenzene yielded the respective product in 88% yield (Table 3, entry 1). Halo substituted phenylacetylenes such as 1-ethynyl-4-fluorobenzene (2c), 1-ethynyl-3-chlorobenzene (2d), and 1-ethynyl-4-bromobenzene (2e) afforded the corresponding ortho-alkenylated products in 78–87% yield (Table 3, entries 2–4). Unfortunately, highly deactivating groups present on aromatic ring of phenylacetylene such as 1-ethynyl-4-nitrobenzene (2f), methyl-4-ethynylbenzoate (2g), 1-ethynyl-4-(trifluoromethyl)benzene (2h) and heteroaromatic alkyne i.e. 2-Ethynylpyridine (2i) were unsuccessful under similar reaction conditions (Table 3, entries 5–7).
| Entry | Ar | Conversionb 1a (%) | Selectivityb 3p–3w (%) | Product; yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (2 mmol), 2b–i (2 mmol), nanosized zeolite beta (100 mg), toluene (1 mL), 120 °C, 3 h, sealed vial.b Conversion and selectivity based on GC.c Isolated yields. | ||||
| 1 | 4-C6H4Me | 94 | 98 | 3p; 88 |
| 2 | 4-C6H4F | 92 | 99 | 3q; 87 |
| 3 | 3-C6H4Cl | 89 | 98 | 3r; 83 |
| 4 | 4-C6H4Br | 83 | 98 | 3s; 78 |
| 5 | 4-C6H4NO2 | 00 | — | 3t; — |
| 6 | 4-C6H4-COOMe | 00 | — | 3u; — |
| 7 | 4-C6H4CF3 | 00 | — | 3v; — |
| 8 | C5H4N | 00 | — | 3w; — |
The reusability of the catalyst is one of the most significant properties for the industrial applications and environmental considerations. The catalyst (nanosized zeolite beta) was easily separated from the reaction mixture by simple filtration. Further, recycling of catalyst was carried out by performing the reaction of aniline with phenylacetylene under standard reaction conditions and the reused catalyst showed consistent activity even after fifth reuse (Table 4). The XRD analysis of reused catalyst matched well with fresh catalyst, thus suggesting that crystallinity of the reused catalyst are comparable to the original material (see the ESI Fig. S1†). There was no leaching of aluminium or silicon from nanosized zeolite beta observed and confirmed by elemental analysis.
| Entry | Cycle | Conversionb 1a (%) | Selectivityb 3a (%) | 3a Yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (2 mmol), 2a (2 mmol), nanosized zeolite beta (100 mg), toluene (1 mL), 120 °C, 3 h, sealed vial.b Conversion and selectivity based on GC.c Isolated yields. | ||||
| 1 | First | 95 | 97 | 90 |
| 2 | Second | 92 | 98 | 88 |
| 3 | Third | 93 | 98 | 89 |
| 4 | Fourth | 90 | 97 | 85 |
| 6 | Fifth | 91 | 99 | 87 |
The plausible reaction mechanism for the ortho-alkenylation of anilines with phenylacetylene over nanosized zeolite beta is illustrated in Scheme 1. It is assumed that alkyne (I) adsorbs on the Bronsted acid sites of zeolite, which subsequently reacts with aniline to give the intermediate II, finally, the resulting intermediate II restoring its aromaticity to produce the respective ortho-alkenylated product III.
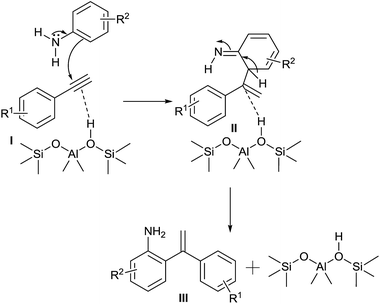 | ||
| Scheme 1 Plausible reaction mechanism for the ortho-alkenylation of anilines with aromatic terminal alkynes. | ||
Conclusions
In summary, a simple and efficient protocol for the ortho-alkenylation of anilines with aromatic terminal alkynes over nanosized zeolite beta has been successfully developed. The scope and limitations of this process are demonstrated with various substituted anilines and aromatic alkynes. However, the substrates (anilines or alkynes) having strong electron-withdrawing groups were unsuccessful with this catalytic system (except 4-nitroaniline). This method demonstrates several notable advantages including use of non-hazardous and reusable catalysts, higher yields of the desired products, high atom economy, simple work-up procedure and mild reaction conditions, which make it an attractive and useful alternative to the existing method.Acknowledgements
We thank the CSIR Network project CSC-0123 for financial support. M. N., C. D. and M. M. R. acknowledge the financial support from CSIR, India in the form of fellowships. P. S. and K. S. acknowledge the financial support from UGC, India in the form of fellowship.Notes and references
- (a) R. M. Roberts and A. A. Khalaf, Friedel–Crafts Alkylation Chemistry, Marcel Dekker, New York, 1984 Search PubMed; (b) R. Taylor, Electrophilic Aromatic Substitution, Wiley, New York, 1990 Search PubMed; (c) H. Heaney, Comprehensive Organic Chemistry, Pergamon, Oxford, 1991, vol. 2, p. 733 Search PubMed; (d) H. Wynberg, Comprehensive Organic Chemistry, Pergamon, Oxford, 1991, vol. 2, p. 769 Search PubMed; (e) O. Meth-Cohn and S. P. Stanforth, Comprehensive Organic Chemistry, Pergamon, Oxford, 1991, vol. 2, p. 777 Search PubMed; (f) H. Ishibashi and M. Ikeda, Rev. Heteroat. Chem., 1996, 14, 59 CAS.
- T. Sugasawa, J. Synth. Org. Chem., Jpn., 1978, 36, 480 CrossRef CAS.
- (a) C. Hausch and G. Helmkamp, J. Am. Chem. Soc., 1951, 73, 3080 CrossRef; (b) C. Hausch, D. G. Crosby, M. Sadoski, A. Leo and D. Percival, J. Am. Chem. Soc., 1951, 73, 704 CrossRef; (c) L. Guo Qiang and N. H. Baine, J. Org. Chem., 1988, 53, 4218 CrossRef.
- (a) R. Smith and T. livinghouse, J. Org. Chem., 1983, 48, 1554 CrossRef CAS; (b) P. D. Magnus and N. L. Sear, Tetrahedron, 1984, 40, 2795 CrossRef CAS; (c) J. B. Jiang, D. P. Hesson, B. A. Dusak, D. L. Dexter, G. J. Kang and E. Hamel, J. Med. Chem., 1990, 33, 1721 CrossRef CAS.
- (a) R. Sarma and D. Prajapati, Chem. Commun., 2011, 47, 9525 RSC; (b) C. Simona, N. J. Clayden, B. Manfred and W. A. Joseph, Chem. Commun., 2009, 7, 797 Search PubMed.
- A. Arienti, F. Bigi, R. Maggi, E. Marzi, P. Moggi, M. Rastelli, G. Sartori and F. Tarantola, Tetrahedron, 1997, 53, 3795 CrossRef CAS.
- (a) A. Dyer, An introduction to zeolite molecular sieves, Wiley, Chichester, 1988 Search PubMed; (b) D. W. Breck, Zeolite molecular sieves, Wiley, New York, 1974 Search PubMed; (c) C. S. Cundy and P. A. Cox, Chem. Rev., 2003, 103, 663 CrossRef CAS PubMed; (d) W. E. Farneth and R. J. Gorte, Chem. Rev., 1995, 95, 615 CrossRef CAS.
- (a) K. Smith, A. Musson and G. A. DeBoos, Chem. Commun., 1996, 469 RSC; (b) G. Bellusi, G. Pazzuconi, C. Perego, G. Girotti and G. Terzoni, J. Catal., 1995, 157, 227 CrossRef; (c) A. M. Camiloti, S. L. Jahn, N. D. Velasco, L. F. Moura and D. Cardoso, Appl. Catal., A, 1999, 182, 107 CrossRef CAS; (d) S. Mintova, V. Valtchev, T. Onfroy, C. Marichal, H. Knozinger and T. Bein, Microporous Mesoporous Mater., 2006, 90, 237 CrossRef CAS PubMed; (e) K. P. de Jong, C. M. A. M. Mesters, D. G. R. Peferoen, P. T. M. van Brugge and C. de Groot, Chem. Eng. Sci., 1996, 51, 2053 CrossRef CAS.
- G. Majano, S. Mintova, O. Ovsitser, B. Mihailova and T. Bein, Microporous Mesoporous Mater., 2005, 80, 227 CrossRef CAS PubMed.
- (a) J. Kecht, B. Mihailvoa, K. Karaghisoff, S. Mintova and T. Bein, Langmuir, 2004, 20, 5271 CrossRef CAS; (b) M. Tsapatsis, M. Lovallo, T. Okubo, M. E. Davis and M. Sadakata, Chem. Mater., 1995, 7, 1734 CrossRef CAS; (c) L. Tosheva and V. P. Valtchev, Chem. Mater., 2005, 17, 2494 CrossRef CAS.
- (a) J. P. Gilson and E. G. Derouane, J. Catal., 1984, 88, 538 CrossRef CAS; (b) J. H. Kim, T. Kunieda and M. Niwa, J. Catal., 1998, 173, 433 CrossRef CAS.
- (a) N. Narender, K. S. K. Reddy, M. A. Kumar, C. N. Rohitha and S. J. Kulkarni, Catal. Lett., 2010, 134, 175 CrossRef CAS; (b) K. V. V. K. Mohan, N. Narender and S. J. Kulkarni, Microporous Mesoporous Mater., 2007, 106, 229 CrossRef PubMed; (c) K. V. V. K. Mohan, N. Narender and S. J. Kulkarni, Green Chem., 2006, 8, 368 RSC; (d) N. Narender, K. V. V. K. Mohan, R. V. Reddy, P. Srinivasu, S. J. Kulkarni and K. V. Raghavan, J. Mol. Catal. A: Chem., 2003, 192, 73 CrossRef CAS; (e) N. Narender, P. Srinivasu, S. J. Kulkarni and K. V. Raghavan, J. Catal., 2001, 202, 430 CrossRef CAS; (f) N. Narender, P. Srinivasu, S. J. Kulkarni and K. V. Raghavan, Green Chem., 2000, 2, 104 RSC; (g) M. Naresh, M. M. Reddy, P. Swamy, M. A. Kumar, K. Srujana, C. Durgaiah and N. Narender, Catal. Commun., 2015, 61, 41 CrossRef PubMed.
- M. M. Reddy, M. A. Kumar, P. Swamy, M. Naresh, K. Srujana, L. Satyanarayana, A. Venugopal and N. Narender, Green Chem., 2013, 15, 3474 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and NMR spectra (1H and 13C). See DOI: 10.1039/c5ra16931c |
| This journal is © The Royal Society of Chemistry 2015 |



