5-Phenyl-dipyrromethane and 5-(4-pyridyl)-dipyrromethane as modular building blocks for bio-inspired conductive molecularly imprinted polymer (cMIP). An electrochemical and piezoelectric investigation†
S. Susmel*a and
C. Comuzzib
aDepartment of Food Science, Analytical Chemistry Group, University of Udine, Via Sondrio 2/A, 33100 Udine, Italy. E-mail: sabina.susmel@uniud.it; Fax: +39 432 558803; Tel: +39 432 558823
bDepartment of Chemistry, Physics and Environment, University of Udine, Via del Cotonificio 108, 33100 Udine, Italy. E-mail: clara.comuzzi@uniud.it; Fax: +39 432 558803; Tel: +39 432 558845
First published on 9th September 2015
Abstract
5-Phenyl-dipyrromethane (5-ph-DP) and 5-(4-pyridyl)dipyrromethane (5-py-DP) are proposed, for the first time, as electroactive building blocks for the preparation of sensors based on molecularly imprinted conductive polymers (cMIP). This paper reports the electrochemical and gravimetric investigation on 5-phenyl-dipyrromethane and 5-(4-pyridyl)dipyrromethane and it demonstrates their ability to form both conductive homo-polymers (cMIP) and co-polymers (co-cMIP). The template salicylic acid (SA) was reversibly and selectively incorporated in the obtained synthetic pockets as proved by both voltammetric and piezoelectric investigation. Moreover, the sensitivity of co-cMIP was higher compared to the two homopolymers. The analytical performances confirm that dipyrromethanes, properly functionalized, can be used as electroactive amino acid-like monomers, to prepare bio-inspired imprinted polymers.
1. Introduction
The development of sensors based on molecularly imprinted polymers (MIP) has received great attention in recent years.1–3 The preparation of MIP requires that the polymer grows around a template (the target analyte) which is embedded in the polymer network in which it draws a cavity defined by size and stereochemical configuration. Once the template molecule is removed, the cavity acts as a 3D recognition unit highly specific for the target analyte.4 In order to reach a high level of efficiency for the entire process, it is essential that the template, interacting reversibly with the monomer, does not deactivate its polymerization positions, this holding true especially when the polymerization is electrochemically driven. Pyrrole is frequently used as electroactive monomer for MIP preparation.5,6 The template inclusion is driven on the basis of a charge interaction process between the electrogenerated aromatic cation and the template in ionic form. Thus so, the process is poorly versatile and it lacks in selectivity requiring the respect of the above conditions to be efficient.7 One of the strategies adopted to enlarge the fields of application is to use “bifunctional” monomers whose structures bear both the unit devoted to the molecular recognition and the unit of polymerization. However, in most cases, the functionalization of the monomer impairs its polymerization features, thus solely some molecular structures can be used as recognition moiety. Consequently, the selectivity cannot be accurately tuned. In the present work the electrochemical behaviour of the 5-phenyl-dipyrromethane (5-ph-DP) and 5-(4-pyridyl)-dipyrromethane (5-py-DP) (Fig. 1) is investigated, for the first time with the aim to assess whether dipyrromethanes can be useful monomers to synthetize electrochemically conductive MIP (cMIP). The interest in these molecules lies in the fact that the recognition unit is located in a remote position with respect to the two pyrrolic units, which act as polymerization sites. Further, the modular synthesis of dipyrromethanes allows the most convenient functionality for the chemical interaction with the template to be easily introduced in position 5. Then a series of differently substituted dipyrromethane can be synthetized and conveniently mixed to obtain highly selective cMIP.In biological receptors, the polypeptidic structure, through the aminoacid-side chains, guarantees the geometrical and chemical complementarity for the specific interaction with ligand. The idea to proof is that a receptor bio-inspired can be reproduced co-polymerising dipyrromethanes differently substituted in C5. From this perspective, functionalised dipyrromethanes, can be accounted as aminoacid-like electroactive monomers, in which the electrogenerated pyrrolic α–α-linkage acts as the peptidic backbone and the substitution in C5 plays the role of the aminoacid-side chains. The aim of this preliminary study is first to investigate the role of the substituent in 5 both on dipyrromethane polymerization ability and on the recognition of the template. Then, the co-polymerization of 5-ph-DP and 5-py-DP is also tested with the goal of demonstrating that multifunctional pockets bearing phenyl and pyridyl pendant groups can be electrochemically obtained. As template prototype of multifunctional ligands the electroactive salicylic acid (SA)8 was chosen.
The electrochemical investigation of the monomers and their polymerization ability is conducted by cyclic voltammetry (CV) and differential pulse voltammetry (DPV), either in a conventional three-electrode cell and at a Pt-quartz crystal electrode in EQCM (Electrochemical Quartz Crystal Microbalance) device. The simultaneous dual signal of current and mass variation recorded by EQCM is used to investigate the growth of the films at the quartz crystal electrode surface. The obtained MIP-modified crystal was then used in QCM (Quartz Crystal Microbalance) modality to investigate the SA uptake/release by gravimetric signal variation. The selectivity of the piezoelectric quartz crystal (QCM) cMIP-modified, is assessed on three interferents, structurally similar to salicylic acid, such as benzoic acid (HBz), phenol (PhOH) and 3-hydroxy-benzoic acid (3-HB). The real samples chosen to test the MIP sensors were the extracts of willow buds. SA is one of the phytohormones involved in plants adaptive responses to the environmental stresses and it is a key signaling molecule in plant defence against biotrophic pathogens.9,10
2. Material and methods
2.1. Materials
Salicylic acid (99%) (SA), tetrabutylammonium perchlorate (TBAP), glacial acetic acid (HAc) and acetonitrile (AN) from Sigma-Aldrich (IT) were all of reagent grade.2.2. Equipment and procedures
Differential pulse voltammetry (DPV) adopted these optimized conditions: pulse amplitude 50 mV; sample width 20 ms; pulse width 50 ms; pulse period 200 ms; sensitivity 1 × 10−5 A V−1.
All investigations were performed by CHI 400C working station (CH Instruments, Inc., Austin, TX, USA).
| Δf = −2f02Δm/[A(μρ)1/2] |
2.3. Synthesis of 5-phenyl-dipyrromethane (5-ph-DP) and 5-(4-pyridyl)-dipyrromethane (5-py-Dp)
5-Phenyl-dipyrromethane (5-ph-DP) and 5-(4-pyridyl)-dipyrromethane (5-py-Dp) were synthesized as reported in literature.12–14 Briefly, pyrrole (7 ml) and benzaldehyde (0.4 ml) were degassed with N2 for 15 min. 0.03 ml of trifluoroacetic acid (TFA) were then added and the reaction mixture stirred for 15 min. Evaporation to dryness and flash chromatography (petroleum ether/ethyl acetate 95/5) gave 5-ph-DP in 61% yield. 4-Pyridinecarboxaldehyde (0.9 ml) was stirred for 18 h at 85 °C with pyrrole (9.1 ml), under nitrogen atmosphere. Evaporation to dryness and alumina flash-chromatography (alumina; ethyl acetate/pentane 8/2) afforded 5-py-Dp in 58%.2.4. NIP and MIP preparation
The polymer deposition was obtained by cycling 17 times the potential between −0.6 V and +1.2 V. This procedure was adopted to obtain both conductive non-imprinted homopolymer (cNIP) and when the template, salicylic acid (SA), was added to the solution, conductive molecularly imprinted homopolymer (cMIP). Monomers were in concentration of 1.0 × 10−3 mol L−1 and SA was 1.5 × 10−3 mol L−1.To prepare copolymers (co-cNIP and co-cMIP), different ratio of monomers 5-ph-DP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5-py-DP = 1
5-py-DP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2, 1
0.2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 were tested. SA was kept constant at the concentration of 1.5 × 10−3 mol L−1 and it was extracted from the imprinted polymers and copolymers by AN added with 1% HAc (v/v) (about 20 min at RT). The modified electrode was equilibrated 10 min at RT in AN + 0.05 mol L−1 TBAP before each measurement. These experimental procedures were adopted either in the investigation performed at Pt-working electrode in a conventional three-electrode electrochemical cell either at Pt-quartz crystal electrode for EQCM/QCM investigations.
0.1 were tested. SA was kept constant at the concentration of 1.5 × 10−3 mol L−1 and it was extracted from the imprinted polymers and copolymers by AN added with 1% HAc (v/v) (about 20 min at RT). The modified electrode was equilibrated 10 min at RT in AN + 0.05 mol L−1 TBAP before each measurement. These experimental procedures were adopted either in the investigation performed at Pt-working electrode in a conventional three-electrode electrochemical cell either at Pt-quartz crystal electrode for EQCM/QCM investigations.
2.5. QCM evaluation of sensor response
To evaluate the uptake/release of the template by QCM, once the cMIP and co-cMIP were prepared, SA was extracted as procedure in Section 2.4 NIP and MIP preparation. The piezoelectric crystal, which was hold in its Teflon cell, was rinsed with fresh solvent and it was equilibrated with 3 ml of a quiescent solution of AN + 0.05 mol L−1 TBAP at RT. When the steady frequency was obtained (f0), SA was added and the variation of the frequency value was monitored to stability (f1). The signal, Δf, calculated as Δf = f1 − f0 was used to graph the calibration curve vs. SA concentration. A range of SA concentration from 1 × 10−8 mol L−1 to 1 × 10−6 mol L−1 was investigated. The calculated Δf was also used to obtain ng from Hz, using 1.34 Hz ng−1 as conversion factor.The cMIP and co-cMIP selectivity was tested vs. 3-hydroxybenzoic acid, phenol and benzoic acid as interferents. To evaluate the aspecific signal of conductive non-imprinted polymer (cNIP) and copolymer (co-cNIP) the same procedures were adopted.
2.6. Real samples
Buds from willows (i.e. Salix alba) were cleaned from scales, frozen with liquid nitrogen than grinded to powder. To extract SA, the procedure optimized was repeated two times on a same sample: 0.10 g of buds powder weight in a Eppendorf vial were added with 1 ml of AN + 1% HAc (v/v), mixed 60 s on a vortex mixer (ThermoFischer-Scientific-IT) and centrifuged for 10 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g (Centrifuge 5804 R-Eppendorf-DE). The supernatant (1 ml) was transferred to a new vial and the extraction procedure was repeated on the pellet of plant sample. The recovery was assessed on 0.10 g of buds powder spiked with 425 μg of SA. Both the buds powder and the extracted supernatant (2 ml) were stored at −20 °C until use. As reference method, the colorimetric detection (at 540 nm) of the violet-blue complex that SA forms with Fe(III) was used.15
000g (Centrifuge 5804 R-Eppendorf-DE). The supernatant (1 ml) was transferred to a new vial and the extraction procedure was repeated on the pellet of plant sample. The recovery was assessed on 0.10 g of buds powder spiked with 425 μg of SA. Both the buds powder and the extracted supernatant (2 ml) were stored at −20 °C until use. As reference method, the colorimetric detection (at 540 nm) of the violet-blue complex that SA forms with Fe(III) was used.15
3. Results and discussion
3.1. Electrochemical investigation on 5-ph-DP and 5-py-DP and salicylic acid
The structures of 5-ph-DP and 5-py-DP and their cyclovoltammograms (CV) recorded at Pt-WE are reported in Fig. 1A and B, respectively. The potential of the anodic peak (Epa) related to the formation of the radical cation was observed, for both monomers, at +1.2 V. A second oxidation process was recorded at +1.5 V for 5-ph-DP and at +1.8 V for 5-py-DP. The apparent number of electrons exchanged (napp) in the first oxidation process was 2.3 e− per molecule for 5-ph-DP and 1.2 e− per molecule for 5-py-Dp. Conversely, the ip of second oxidative process of the two dipyrromethanes was comparable. CVs at different scan rate were registered (see ESI Fig. S1 and S2†) showing that the radical cation was stabilized by the presence of phenyl (ph) group in C5. At 300 mV s−1 a quasi-reversible process for 5-ph-DP was observed (reversibility in bipyrrole required a scan rate of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 V s−1),16,17 while, no pseudo-reversibility was observed for 5-py-DP. The basicity of pyridine on the carbocation stability could be responsible of these experimental evidences. Tacking into account the literature reported “pyridine effect” on pyrrole electrochemistry,16–23 the cyclic voltammetry of the N-methylated 5-py-DP derivative was performed. The aim was to ascertained if the halve ip observed in 5-py-DP, respect to 5-ph-DP, was due to a reaction of pyridyl-substituent with the electrogenerated carbocation on dipyrromethane moiety. The quaternization of the pyridine nitrogen causes the displacement of the first oxidation wave toward more anodic potential (data not shown). The ip of the first oxidation wave was unchanged suggesting that pyridine does not affect the radical cation formation,24,25 however, ip of the second process was halving. CV of 5-py-DP in the presence of tosylate, as stabilizer of radical cation and cationic species through ion-paring,18 was also recorded (see ESI Fig. S3†). It was observed that, increasing the amount of tosylate in solution, ipa of both oxidation processes (at +1.2 V and +1.8 V) increased while their Epa became less anodic. Tosylate anion can sterically form ion-pair with –NH+s generated during the oxidation process. The shielding of the positive charge is responsible for the cathodic shift of both oxidation processes. Moreover, ip related to the radical-cation formation of pyridyl-derivative turns to napp = 2. These data suggest that the low ipa observed (Fig. 1A and B) for the first oxidation wave of 5-py-DP is related to the different stability of its radical cation. The effect of pyridyl-group is observed just on the process at 1.8 V. On the basis of the mechanism scheme proposed by Zotti et al.18 for 2,2′-bipyrrole, the hypothesis was formed that pyridine (pKa of pyridine in AN = 16.7
000 V s−1),16,17 while, no pseudo-reversibility was observed for 5-py-DP. The basicity of pyridine on the carbocation stability could be responsible of these experimental evidences. Tacking into account the literature reported “pyridine effect” on pyrrole electrochemistry,16–23 the cyclic voltammetry of the N-methylated 5-py-DP derivative was performed. The aim was to ascertained if the halve ip observed in 5-py-DP, respect to 5-ph-DP, was due to a reaction of pyridyl-substituent with the electrogenerated carbocation on dipyrromethane moiety. The quaternization of the pyridine nitrogen causes the displacement of the first oxidation wave toward more anodic potential (data not shown). The ip of the first oxidation wave was unchanged suggesting that pyridine does not affect the radical cation formation,24,25 however, ip of the second process was halving. CV of 5-py-DP in the presence of tosylate, as stabilizer of radical cation and cationic species through ion-paring,18 was also recorded (see ESI Fig. S3†). It was observed that, increasing the amount of tosylate in solution, ipa of both oxidation processes (at +1.2 V and +1.8 V) increased while their Epa became less anodic. Tosylate anion can sterically form ion-pair with –NH+s generated during the oxidation process. The shielding of the positive charge is responsible for the cathodic shift of both oxidation processes. Moreover, ip related to the radical-cation formation of pyridyl-derivative turns to napp = 2. These data suggest that the low ipa observed (Fig. 1A and B) for the first oxidation wave of 5-py-DP is related to the different stability of its radical cation. The effect of pyridyl-group is observed just on the process at 1.8 V. On the basis of the mechanism scheme proposed by Zotti et al.18 for 2,2′-bipyrrole, the hypothesis was formed that pyridine (pKa of pyridine in AN = 16.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26), acting as a H+ scavenger, drags the reaction toward the product of coupling reaction, helping the deprotonation step necessary for the restoration of conjugation on dipyrromethane22–25 (Scheme 1, eqn (3)).15–17,22–25
26), acting as a H+ scavenger, drags the reaction toward the product of coupling reaction, helping the deprotonation step necessary for the restoration of conjugation on dipyrromethane22–25 (Scheme 1, eqn (3)).15–17,22–25
The electroactive template was solubilized in acetonitrile (solubility 0.35 mol L−1) and its CV profile is reported in Fig. 2A and B together with the voltammetric profile of 5-ph-DP and 5-py-DP. The oxidation of the phenolic group is observed at +2.1 V, its reduction in the reverse cycle at +0.6 V, and the cathodic reduction of the carboxylic proton at −0.5 V. The interactions between monomers and template were investigate recording the first CV cycle separately either in the anodic and the cathodic direction in N2 bubbled solution. As increasing amounts of SA were added to a 1 mM solution of 5-ph-DP (Fig. 2A), the oxidation process of –OH increased proportionally, while Epa shifted to more anodic potential suggesting the interaction with the dipyrromethane structure. For 5-py-DP (Fig. 2B), the SA–OH interaction with the structure of the monomer was much stronger as its oxidation was not visible except when in large excess compared to the concentration of the monomer (at least 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The cathodic part of the cyclovoltammogram of both monomers, showed a less defined reduction process shifted of 150 mV toward more cathodic voltage, indicating the interaction of SA–COOH group with dipyrromethanes.
1). The cathodic part of the cyclovoltammogram of both monomers, showed a less defined reduction process shifted of 150 mV toward more cathodic voltage, indicating the interaction of SA–COOH group with dipyrromethanes.
3.2. Electrochemical investigations of 5-ph-DP and 5-py-Dp cNIP and cMIP. Homo- and co-polymers
The polymerization ability of both monomers was investigated by EQCM. cNIP and cMIP of homo- and co-polymers formation at Pt-quartz crystal-WE was performed by cyclic voltammetry. The experimental parameters, such as potential limits, scan rate and number of cycles were varied to optimize the film coating. The best potential range was between −0.6 and +1.2 V at a scan rate 0.1 V s−1. During film deposition of homo-5-ph-DP (1 × 10−3 mol L−1 in AN + TBAP 0.05 mol L−1), the current increased progressively and the film formed between +0.3 V and +0.4 V giving a cNIP surface coverage (Γ) of 3.42 × 10−9 mol cm−2 (Fig. 3A). The film thickness increased of 2.7 nm per cycle showing a linear relation with the cycle numbers as esteemed by profilometry. Also the progressively more negative frequency shift (see ESI Fig. S4†), showed that the film increased regularly its thickness both during the anodic and the reverse scan up to E = 0 V. Then, in the cathodic part of the scan (Ep < 0 V), at each cycle a constant mass release of about 3% was observed. This behavior has been typically observed in conductive polymers with ion exchange capacity, such as polypyrrole.5The same conditions were used to polymerize 5-py-DP (1 × 10−3 mol L−1 in AN + TBAP 0.05 mol L−1). In this case cNIP grown at lower potential between −0.2 V and +0.2 V (Γ = 2.45 × 10−9 mol cm−2) (Fig. 4A). However, after several cycles the efficiency of deposition process dropped down (mass increases only 0.01% per cycle) (Table 1). The profiles of frequency shift (see ESI Fig. S5†) showed that the mass increase of poly-5-py-DP was confined just to the anodic part of the scan. These evidences suggested that the charge transport in poly-5-py-DP, involving the electrolyte added to the solvent, might be affected by the increasing concentration at the electrode surface of protonated 5-py-DP (see Scheme 1), that finally impairs the film conductivity.22
| 5-ph-DP | 5-py-DP | Co-polymer 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||||||
|---|---|---|---|---|---|---|---|---|
| Cycle number | cNIP(1) ng per cycle | cMIP(2) ng per cycle | Cycle number | cNIP(3) ng per cycle | cMIP(4) ng per cycle | Cycle number | cNIP(5) ng per cycle | cMIP(6) ng per cycle |
| 1 | 58.96 | 109.21 | 1 | 53.6 | 80.4 | 1 | 187.6 | 250.58 |
| 2 | 168.84 | 178.622 | 2 | 53.6 | 67.0 | 2 | 235.84 | 320.26 |
| 3 | 188.94 | 206.896 | 3 | 40.2 | 60.3 | 3 | 235.84 | 301.5 |
| 4 | 182.24 | 223.78 | 4 | 33.5 | 46.9 | 4 | 233.16 | 294.8 |
| 5 | 198.32 | 230.48 | 5 | 26.8 | 40.2 | 5 | 235.84 | 296.14 |
| 6 | 188.94 | 257.28 | 6 | 13.4 | 26.8 | 6 | 231.82 | 298.82 |
| 7 | 195.64 | 280.06 | 7 | 13.4 | 26.8 | 7 | 235.84 | 301.5 |
Homo-cMIP of both 5-ph-DP and 5-py-DP was formed through the SA inclusion into the polymer network which is thought of occurring through several types of attractive interactions such as π–π interactions between aromatic rings, hydrogen bridges between –OH and –COOH of salicylic acid and N-of dipyrromethane and/or pyridyl-group in the case of 5-py-DP, charge interactions between the dipyrromethane radical-cation and salicylate in equilibrium with salicylic acid. Homo-cMIPs were prepared using equimolar solution of monomer and template (1 mM in AN + 0.05 mol L−1 TBAP) and the same voltage limits and scan rate used in cNIP preparation. CV, DPV and gravimetric data (QCM) were used to demonstrate the inclusion of the template into the films formed and the rebinding ability of the imprinted polymer. The CV of 5-ph-DP cMIP (Fig. 3B) showed two new peaks compared to the CV of 5-ph-DP cNIP (Fig. 3A): the process at −0.4 V, ascribed to the reduction of SA–COOH interacting with 5-ph-DP, and, in oxidation, a new wave at +0.9 V, both rising during deposition. The anodic current at +1.2 V, at which the radical cation was formed, increased as well during the deposition confirming that the polymer–template interactions do not affect the formation of the radical cation. EQCM measurements clearly confirmed the uptake of SA. Table 1 reports the mass variation at each cycle of film deposition. The mass variation of 5-ph-DP cNIP becomes constant after the second cycle while, for 5-ph-DP cMIP, the mass variation continues to grow indicating the SA uptake. It has to be stressed that both poly-5-ph-DP cNIP and cMIP were conductive during the deposition process suggesting that the inclusion of the electroactive template is not interfering with the polymer growth.27
The DPV (see ESI Fig. S4†) of 5-ph-DP cNIP and cMIP were also recorded showing a slight difference between the two homo-polymer. Interestingly the DPV of cMIP after washing and re-exposing the sensor to a SA solution, was recovered.
The effect of the numbers of cycles on the extraction of the template from the imprinted polymer was then investigated. The extraction procedure was optimized for a 25 cycles film deposition which traps 2.75 × 10−9 mol cm−2 (n = 5, CV% = 10) of SA, as esteemed both by voltammetric and gravimetric data. The best extraction procedure was conditioning the cMIP in AN + TBAP 0.05 mol L−1 added with 1% (v/v) of acetic acid at open circuit potential for 20 min at room temperature. The solvent acidification increased the SA solubility and helped to break off the interactions established with the polymer matrix.
The inclusion of SA into 5-py-DP polymer was then investigated. The CV of 5-py-DP cMIP (Fig. 3B) shows a growing peak at −0.6 V, again ascribed to the reduction of SA–COOH interacting with the polymer, which is totally absent in the CV of 5-py-DP cNIP (Fig. 3A). In oxidation however a non-conducting behavior was observed. EQCM measurements of 5-py-DP cMIP deposition (Table 1) shows a loss of the mass variation with the increase of the cycles, due to the dropping of conductivity of the 5-py-DP film. The difference in mass deposition between 5-py-DP cMIP and cNIP is constant at 13.4 ng at each cycle corresponding to a constant uptake of 0.1 nmol of SA per cycle. The acidic character of the polymeric structure permanently charged at the protonated pyridin-moiety is considered to influence the diffusion of both the electroactive template and fresh monomer toward the electrode surface.19,21,27
The DPV (see ESI Fig. S5†) of 5-py-DP cNIP and cMIP were also recorded. The non-imprinted polymer was characterized by a double wave (at 1.1 V and 1.6 V) of comparable current density. In the DPV of the imprinted polymer a peak at Ep +1.4 V was observed whose current density varied when SA was extracted and rebounded to cMIP. The DPVs profiles of both cNIP of 5-ph-DP and 5-py-DP were unchanged after incubation in SA (1 mM) solution. This evidence corroborated that unspecific interactions were not detectable.
Finally, the co-polymer was prepared. The threshold potential for the initiation of polymerization of the two monomers are very close to each other rendering their copolymerization feasible.28–30
The first scan of the CV of an equimolar (1 × 10−3 mol L−1 mM in AN) solution of the two monomers, showed the formation of radical cations as a well-defined peak at +1.1 V (Fig. 5A). The anticipation of 100 mV of the first oxidation process indicates the interaction between the two monomers. In the following cycles, the evolution of the CV profile with a new oxidation wave at +0.8 V suggests the formation of a copolymer which increased between +0.3 V and +0.5 V. Some features of both homo cNIPs (Fig. 3A and 4A) can be recognized in Fig. 5A, i.e. the potential range of film growing and the current intensity are close to the one of 5-ph-DP cNIP. However, the strong indication of the presence of py-derivative in the film is that the deposition, after few cycles becomes less efficient likewise homo-poly-5-py-DP (Table 1). EQCM frequency shift (see ESI Fig. S6†), is affected by the effect of py-derivative as the deposition of co-cNIP was confined to the anodic part of the scans. Interestingly, in the earlier scans, the co-polymer deposits better than homo polymers suggesting a cooperative effect between monomers.
co-cMIP preparation was investigated adding 1.5 × 10−3 mol L−1 of SA to a monomer mixture (1 × 10−3 mol L−1 5-ph-DP + 1 × 10−3 mol L−1 5-ph-DP) (Fig. 5B). The template inclusion was confirmed by CV where the pre-wave at +0.9 V is observed as in poly-5-ph-DP (Fig. 3B). The increment of the cathodic current in the next cycles is also an indication of SA uptake. The SA inclusion was demonstrated by Δf (Hz) variation as the mass deposited at each cycle increased, compared to co-cNIP, of ca. 26% (Table 1), corresponding to 0.5 nmol per cycle of SA uptaken.
The Ep and ip observed in DPV of co-cNIP (see ESI Fig. S6†) indicated the presence of both monomers at the modified surface. In co-cMIP the inclusion was suggested by the higher current density of the two oxidation processes at 1.1 V and 1.5 V shifted to slightly more anodic voltage (vs. co-cNIP).
Table 2 reports the mass variation after 17 cycles of different monomers feed ratio. It is possible to observe that as the py-monomer concentration increased, the cooperative uptake ability of the MIP increased as well. 5-py-DP enhances SA uptake in co-cMIP participating actively in the binding pocket. Moreover, the extraction time of the template from co-cMIP was doubled respect to homo-phenyl-cMIP due to stronger attractive interactions between pyridyl-moiety and SA. On the other hand, this trend stopped when the concentration of 5-py-DP became prevalent with respect to the concentration of the ph-derivative as a consequence of its interference on the polymerization process.21,22
| 5-py-DP (mM) | 5-ph-DP (mM) | SA (mM) | Δf (Hz) |
|---|---|---|---|
| 0.6 | 0 | 0 | −236.3 (±4.0) |
| 0 | 0.6 | 0 | −1045.2 (±3.8) |
| 0.6 | 0.6 | 0 | −1118.2 (±5.6) |
| 0.12 | 0.6 | 1.5 | −1293.3 (±8.0) |
| 0.3 | 0.6 | 1.5 | −1320.3 (±5.2) |
| 0.45 | 0.6 | 1.5 | −1423.8 (±4.9) |
| 0.6 | 0.6 | 1.5 | −1514.3 (±5.4) |
| 1.2 | 0.6 | 1.5 | −246.3 (±6.5) |
The rebinding ability of co-cMIP was evaluated by QCM (Fig. 6). co-cMIP prepared from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 monomer feed ratio shows the best rebinding performances and it was then chosen as sensor for the analytical characterization.
1 monomer feed ratio shows the best rebinding performances and it was then chosen as sensor for the analytical characterization.
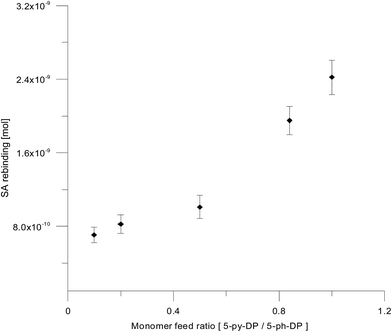 | ||
| Fig. 6 Effect of the monomer feed ratio on the SA rebinding. All polymers obtained by cyclic voltammetry (17 cycles) at Pt-EQCM quartz crystal WE vs. Ag/AgCl, Clsat− in AN + 0.05 M TBAP. | ||
3.3. QCM evaluation of sensor response
The sensor response was evaluated by QCM. The 3D-structure of the cavity left into the polymeric structure is able to rebind selectively the SA added in solution as analyte. A typical trend of the frequency shift observed during the SA rebinding studies at co-cMIP is reported in Fig. 7. The addition of different concentrations of SA produced an immediate variation of the crystal oscillation frequency and the response time evaluated at 90% of Δf was ca. 50 s for all cMIP. The template removal from co-cMIP restored the initial Hz value. | ||
| Fig. 7 co-cMIP response at different total concentration of SA in solution: (a) 1.5 × 10−7 M, (b) 3.0 × 10−7 M, (c) 4.5 × 10−7 M, (d) 5.5 × 10−7 M, (e) 0.7 × 10−7 M, (f) 1.5 × 10−7 M. | ||
The rebinding ability of homo and co-cMIPs prepared from different monomer feed ratio is reported in Fig. 8 as Δf vs. concentration of SA (mol L−1) added in solution. All cMIP considered showed a dynamic range between 10−8 mol L−1 and 10−6 mol L−1 of SA while the linear range is observed between 10−8 mol L−1 and 10−7 mol L−1. The detection limits calculated as 3σ/S were reported in Table 3. Before the SA addition, cMIP was left to equilibrate in solvent. Once the frequency shift was stabilised, the scattering (ΔHz) recorded during 60 s was used to estimate the standard deviation (σ) of the signal.
Poly-5-py-DP gives the lower response in trend with its low polymerization ability with a maximum shift of ca. −100 Hz (CV = 18%, n = 3) while poly-5-ph-DP rebinds almost the double of SA. The synergic effect of co-polymers is evidenced in Table 3 where the sensitivity of detection obtained with polymers of different composition is compared. A higher MIP response is produced rising the amount of py derivative in the feed ratio confirming that the monomers feed ratio affects the concentration of the rebinding sites in the imprinted film, modulating its recognition properties. This synergic effect is the result of combining the better polymerization efficiency of 5-ph-DP and the stronger interaction between the SA and the 5-py-DP. The aspecific interaction with non-imprinted polymer was evaluated exposing c-NIP and co-cNIP to different SA concentrations (Fig. 8). In all the cNIP the higher shift recorded was ca. −80 Hz irrespective of the variation of the SA concentration.
Compounds structurally similar to SA, such as 3-hydroxy benzoic acid (3-HB), phenol (PhOH) and benzoic acid (HBz), were selected to test the selectivity of the co-cMIP sensor. co-cMIP was exposed to a 3 × 10−7 mol L−1 solution of interferent. Results shown in Fig. 9 highlight that a selective response was obtained. The sensor uptakes PhOH and HBz in comparable amounts suggesting that both the hydroxyl and the carboxylic functions are involved in the network of non-covalent interactions established inside the recognition pocket. However when these two functions are present on the same molecule but in a different position respect to the template, as in 3-HB, the sensor response decreased by a half. Consequently co-cMIP shown the higher selectivity vs. 3-HB, the meta isomer of SA. The data confirm that the geometric fitting, beside the non-covalent interactions, between substrate and co-cMIP recognition pocket is a key factor in determining the sensor performances in term of response and selectivity. These different behaviors demonstrate also that a pocket was obtained. In the hypothesis of an interaction to the surface due to copolymerized pyridine-moiety, it is believed that not obvious differences in the signal provided by the analysed interferents would been seen.
 | ||
| Fig. 9 co-cMIP response as Δf (Hz) due to SA and interferents molecules, 3-hydroxybenzoic acid (3-HB), phenol (PhOH) and benzoic acid (HBz). All species were 3 × 10−7 mol L−1 in AN + 0.05 M TBAP. | ||
3.4. co-cMIP tests in real samples
Finally, the SA extracted from spiked buds was esteemed by colorimetric approach (0.0135 mol L−1; recovery of 88%).10 The optimized extraction was performed in AN containing acetic acid. The 1% (v/v) of HAc gives both the best extraction performances and the advantage of using the extraction solvent without further manipulation except the dilution. In fact, to avoid saturation of the polymeric film, a concentration of 3 × 10−7 mol L−1 of the extract was added to the QCM-co-cMIP and QCM-co-cNIP modified surfaces. On the basis of the measured Δf, an SA concentration of 2.26 × 10−7 mol L−1 was esteemed by co-cMIP (recovery of 75%; CV = 11%; n = 3) and co-cNIP measured an aspecific signal of 7.31 × 10−8 mol L−1 (CV = 14%; n = 3).4. Conclusions
5-ph-DP and 5-py-DP are interesting bifunctional monomers for cMIP construction thanks to the substituent in 5 which interacts with the template during film formation. In the copolymerized MIP a synergistic effect of the monomers was observed so during the rebinding tests an increased sensitivity was measured respect to those obtained when the monomers were individually polymerized. However, more insight would be posed on the role of the py-derivative on the polymerization of the copolymer. This especially because py-moiety is able to establish attractive interactions with a wide range of organic and inorganic compounds. Moreover, these first evidences seem to strongly support our idea that a family of dipyrromethane differently functionalized in C5 can be synthetized and conveniently used to tune the recognition ability of the cMIP toward the template.References
- Y. Lattach, P. Archirel and S. Remita, J. Phys. Chem. B, 2012, 116, 1467 CrossRef CAS PubMed.
- F. Aboufazeli, H. R. L. Z. Zhad, O. Sadeghi, M. Karimi and E. Najafi, J. AOAC Int., 2014, 97, 173 CrossRef CAS.
- L. Uzun and A. P. F. Turner, Biosens. Bioelectron., 2015 DOI:10.1016/j.bios.2015.07.013.
- S. Li, Y. Ge, S. A. Piletsky and J. Lunec, Molecularly Imprinted Sensors, Elsevier, Amsterdam, 1st edn, 2012 Search PubMed.
- A. Mehdinia, M. O. A. Zanjani, M. Ahmadifar and A. Jabbari, Biosens. Bioelectron., 2013, 39, 88 CrossRef CAS PubMed.
- S. P. Ozkorucuklu, Y. Sahin and G. Alsancak, Sensors, 2008, 8, 8463 CrossRef CAS PubMed.
- M. J. Whitcombe, I. Chianella, L. Larcombe, S. A. Piletsky, J. Noble, R. Porter and A. Horgan, Chem. Soc. Rev., 2011, 40, 1547 RSC.
- A. Torriero, J. M. Luco, L. Sereno and J. Raba, Talanta, 2004, 62, 247 CrossRef CAS PubMed.
- M. Ashraf, N. A. Akram, R. N. Arteca and M. R. Fooland, Crit. Rev. Plant Sci., 2010, 29, 162 CrossRef CAS PubMed.
- G. Marek, R. Carver, Y. Ding, E. Sathyanarayan, X. Zhang and Z. Mou, Plant Methods, 2010, 6, 21 CrossRef PubMed.
- G. Z. Sauerbay, Z. Phys., 1959, 155, 206 CrossRef.
- J. W. Ka and C. H. Lee, Tetrahedron Lett., 2000, 41, 4609 CrossRef CAS.
- R. P. Briñas and C. Brückner, Synlett, 2001, 3, 442 Search PubMed.
- B. J. Littler, Y. Ciringh and J. S. Lindsey, J. Org. Chem., 1999, 64, 2864 CrossRef CAS PubMed.
- C. S. Warrier, M. Paul and M. V. Vineetha, Genetics and Plant Physiology, 2013, 3, 90 Search PubMed.
- G. Bidan and M. Guglielmi, Synth. Met., 1986, 15, 49 CrossRef CAS.
- L. Guyard, P. Hapiot and P. Neta, J. Phys. Chem. B, 1997, 101, 5698 CrossRef CAS.
- G. Zotti, G. Schiavon, S. Zecchin, F. Sannicolò and E. Brenna, Chem. Mater., 1995, 7, 1464 CrossRef CAS.
- C. P. Andreiux, P. Audebert, P. Hapiot and J. Saveant, Synth. Met., 1991, 41, 2877 CrossRef.
- R. J. Waltman and J. Bargon, Can. J. Chem., 1986, 64, 76 CrossRef CAS.
- M. G. Cross, D. Walton, J. Morse, R. J. Mortimer, D. R. Rosseinsky and D. J. Simmonds, J. Electroanal. Chem., 1985, 189, 389 CrossRef CAS.
- P. N. Bartlett, I.-Y. Chung and P. Moore, Electrochim. Acta, 1990, 35, 1273 CrossRef CAS.
- A. F. Diaz, A. Martinez and K. K. Kanazawa, J. Electroanal. Chem., 1981, 130, 181 CrossRef CAS.
- G. R. Mitchell, F. J. Davis and C. H. E. Legge, Synth. Met., 1988, 26, 247 CrossRef CAS.
- K. J. Wynne and G. B. Street, Macromolecules, 1985, 18, 2361 CrossRef CAS.
- F. Eckert, I. Leito, I. Kaljurand, A. Kutt, A. Klamt and M. Deidenhofen, J. Comput. Chem., 2009, 30, 799 CrossRef CAS PubMed.
- L. Özcan and Y. Şahin, Sens. Actuators, B, 2007, 127, 362 CrossRef PubMed.
- K. Dhanalakshmi and R. Saraswathi, J. Mater. Sci., 2001, 36, 4107 CrossRef CAS.
- K. K. Kanazawa, A. F. Diaz, M. T. Krounbi and G. B. Street, Synth. Met., 1981, 4, 119 CrossRef CAS.
- S. Kuwabata, S. Ito and H. Yoneyama, J. Electrochem. Soc., 1988, 135, 1691 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16129k |
| This journal is © The Royal Society of Chemistry 2015 |







